Preparation method of novel ester group organic silicon quaternary ammonium salt
An organosilicon quaternary ammonium salt and ester-based technology is applied in the field of preparation of ester-based organosilicon quaternary ammonium salts, and can solve the problems of difficult biodegradation, low conversion rate of ester amines, environmental pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
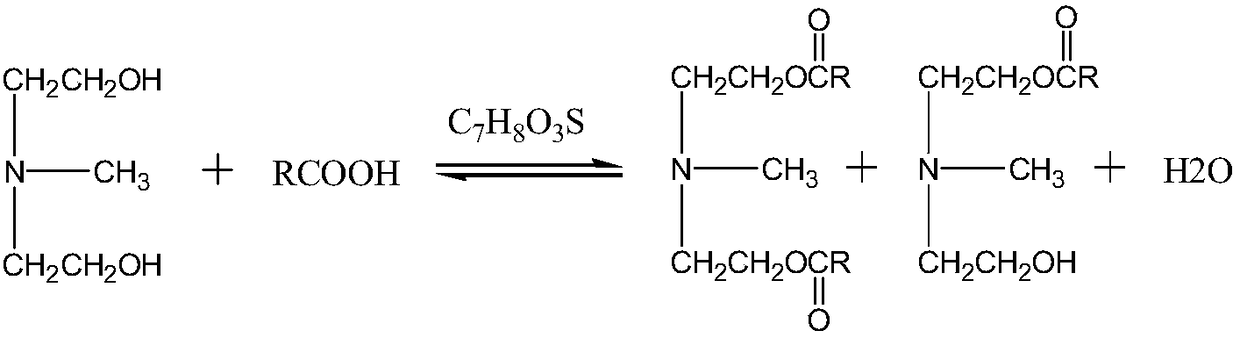
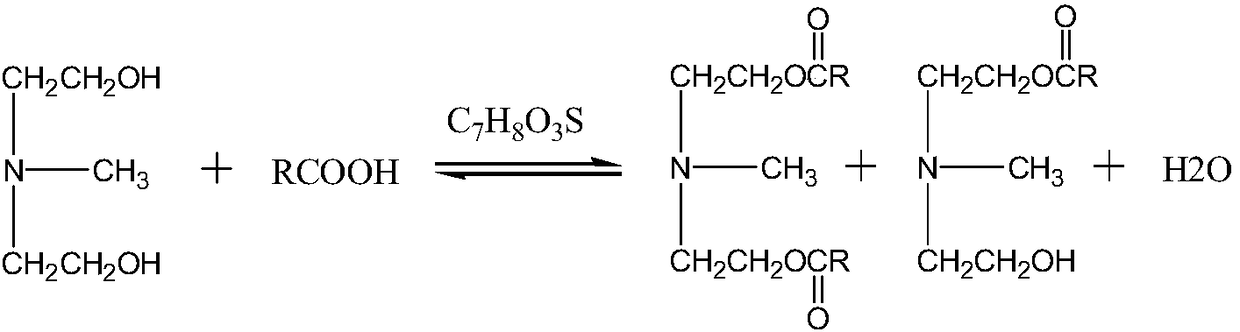
[0011] The present invention provides novel ester group organosilicon quaternary ammonium salt: the synthetic method of methyldiethanolamine laurate mono(bis) ester group propyl trialkoxysilyl ammonium chloride, the method comprises two steps:
[0012] 1) Synthesis of methyldiethanolamine laurate mono(double) ester
[0013] Carry out esterification reaction by N-methyldiethanolamine and lauric acid, take p-toluenesulfonic acid as catalyst;
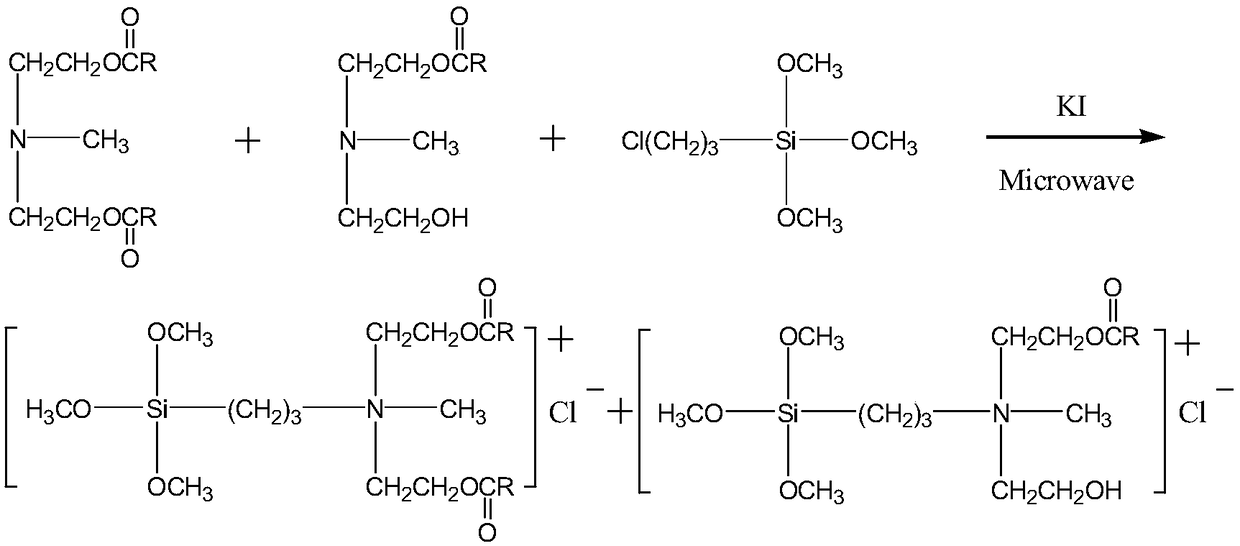
[0014] 2) Synthesis of methyldiethanolamine laurate mono(bis)esteryl propyl trialkoxysilyl ammonium chloride:
[0015] Methyldiethanolamine laurate (double) ester and γ-chloropropyltrialkoxysilane were subjected to quaternization reaction, and methyldiethanolamine laurate was synthesized by microwave method with DMF as solvent and potassium iodide as catalyst. Bis) Ethoxypropyltrialkoxysilyl Ammonium Chloride.
[0016] The γ-chloropropyl trialkoxysilane is γ-chloropropyl trimethoxysilane or γ-chloropropyl triethoxysilane, and the lauric ...
Embodiment 1
[0023] 1. Synthesis of methyldiethanolamine laurate
[0024] Weigh the lauric acid (C 12 h 24 o 2 ), N-methyldiethanolamine (C 5 h 13 NO 2 ), the catalyst p-toluenesulfonic acid (C 7 h 8 o 3 S·H 2 O), join in the there-necked flask. Of which: lauric acid (C 12 h 24 o 2 ) and N-methyldiethanolamine (C 5 h 13 NO 2 ) in a molar ratio of 1.6:1, p-toluenesulfonic acid (C 7 h 8 o 3 S·H 2 O) and lauric acid (C 12 h 24 o 2 ) in a mol ratio of 0.008:1, magnetic stirring, and passing N 2 Protection, oil bath heating reaction, reaction temperature 170 ° C, reaction time 6 hours, the conversion rate of lauric acid reaches 99%; the esterification reaction equation is as follows:
[0025]
[0026] R=C in the above formula 11 h 23 ;
[0027] 2. The synthesis of lauric acid methyldiethanolamine mono(double) ester group propyl trimethoxysilyl ammonium chloride will react the product that obtains in the first step, add solvent N successively, N-dimethyl formamide, c...
Embodiment 2
[0032] 1. Synthesis of methyldiethanolamine laurate
[0033] Weigh the lauric acid (C 12 h 24 o 2 ), N-methyldiethanolamine (C 5 h 13 NO 2 ), the catalyst p-toluenesulfonic acid (C 7 h 8 o 3 S·H 2 O), join in the there-necked flask. Of which: lauric acid (C 12 h 24 o 2 ) and N-methyldiethanolamine (C 5 h 13 NO 2 ) in a molar ratio of 1.6:1, p-toluenesulfonic acid (C 7 h 8 o 3 S·H 2 O) and lauric acid (C 12 h 24 o 2 ) in a mol ratio of 0.008:1, magnetic stirring, and passing N 2 Protection, oil bath heating reaction, reaction temperature 170 ° C, reaction time 6 hours, the conversion rate of lauric acid reaches 99%; the esterification reaction equation is as follows:
[0034]
[0035] R=C in the above formula 11 h 23 ;
[0036] 2. Synthesis of methyldiethanolamine laurate mono(double) ester group propyl triethoxysilyl ammonium chloride
[0037] The product obtained in the first step reaction is sequentially added into solvent N, N-dimethylformamide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com