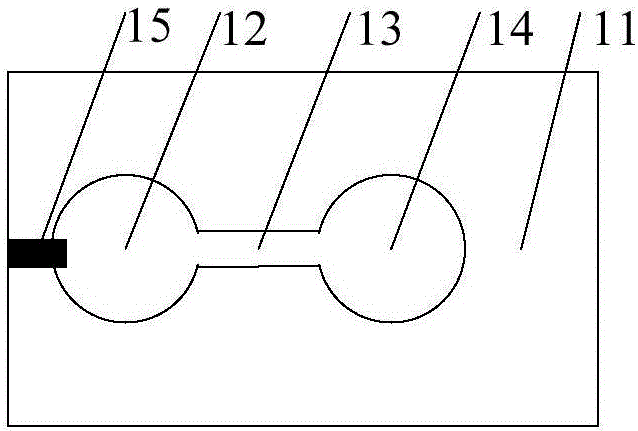Low abundance hormone detection method and device
A low-abundance, hormone-based technology, applied in measurement devices, biological tests, material inspection products, etc., can solve the problems of not being easy to use, high price, physical damage, etc., and achieve the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0056] The preferred embodiments of the present invention will be described in detail below in conjunction with the accompanying drawings. It should be understood that the preferred embodiments described below are only used to illustrate and explain the present invention, and are not intended to limit the present invention.
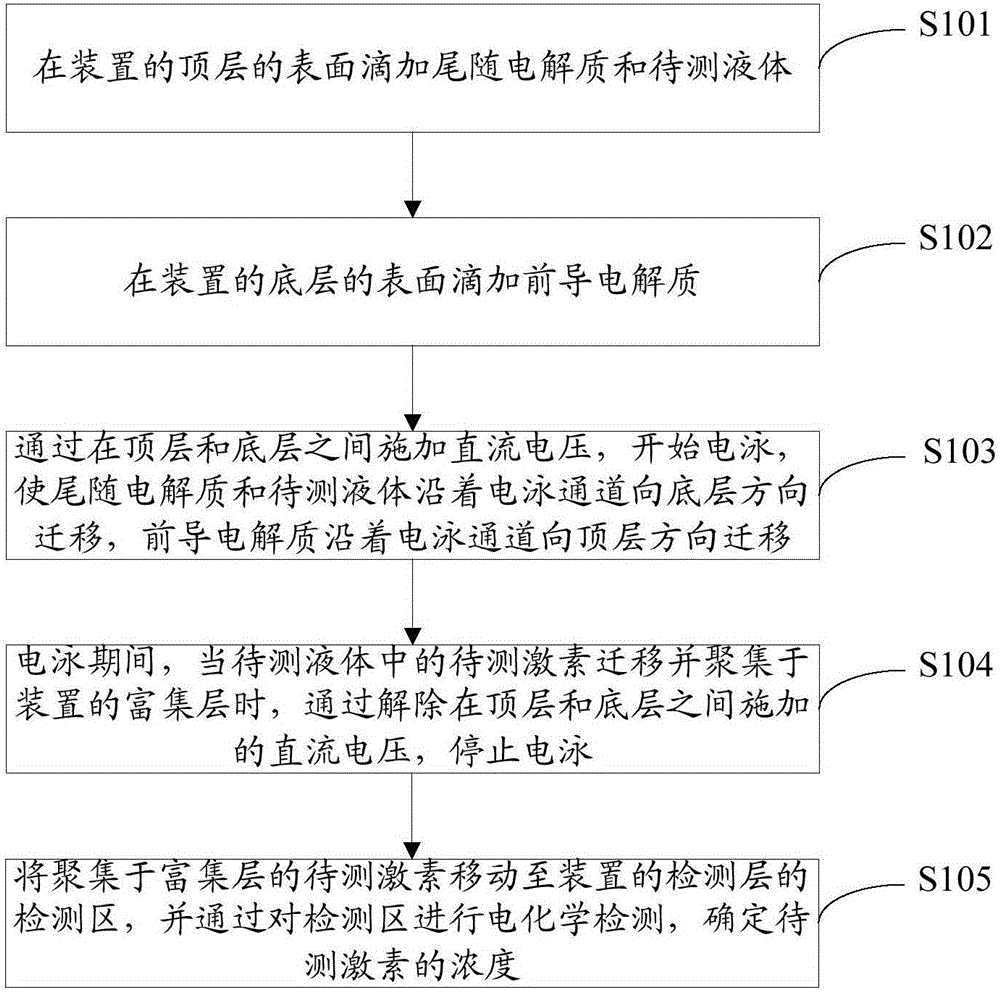
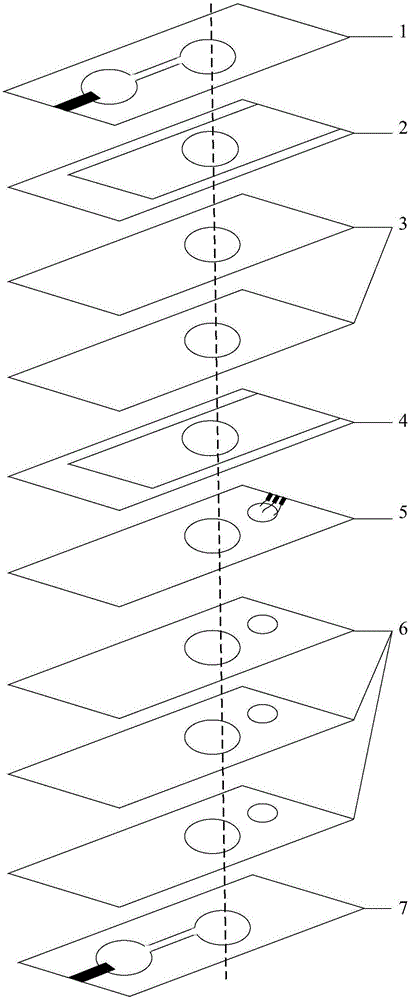
[0057] figure 1 It is a block diagram of the low-abundance hormone detection method provided by the embodiment of the present invention. The detection device has a multi-layer structure including a top layer, an enrichment layer, a detection layer, and a bottom layer. The electrophoretic pathway areas of each layer structure are connected to each other to form an electrophoretic pathway. Such as figure 1 As shown, the steps include:
[0058] Step S101: Dropping the trailing electrolyte and the liquid to be tested on the surface of the top layer of the device.
[0059] The top layer includes a top sample loading layer with a top electrophoretic electrode...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com