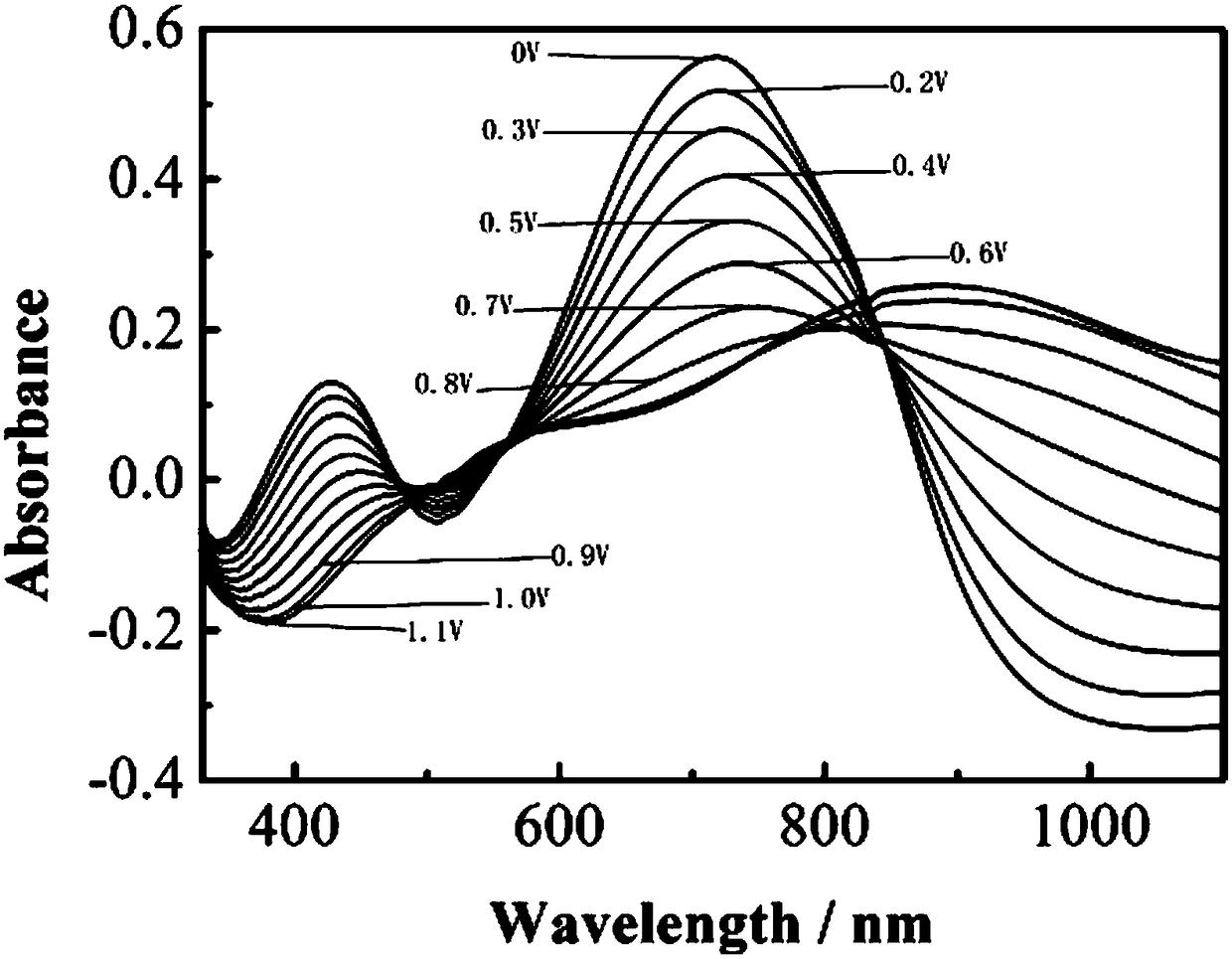
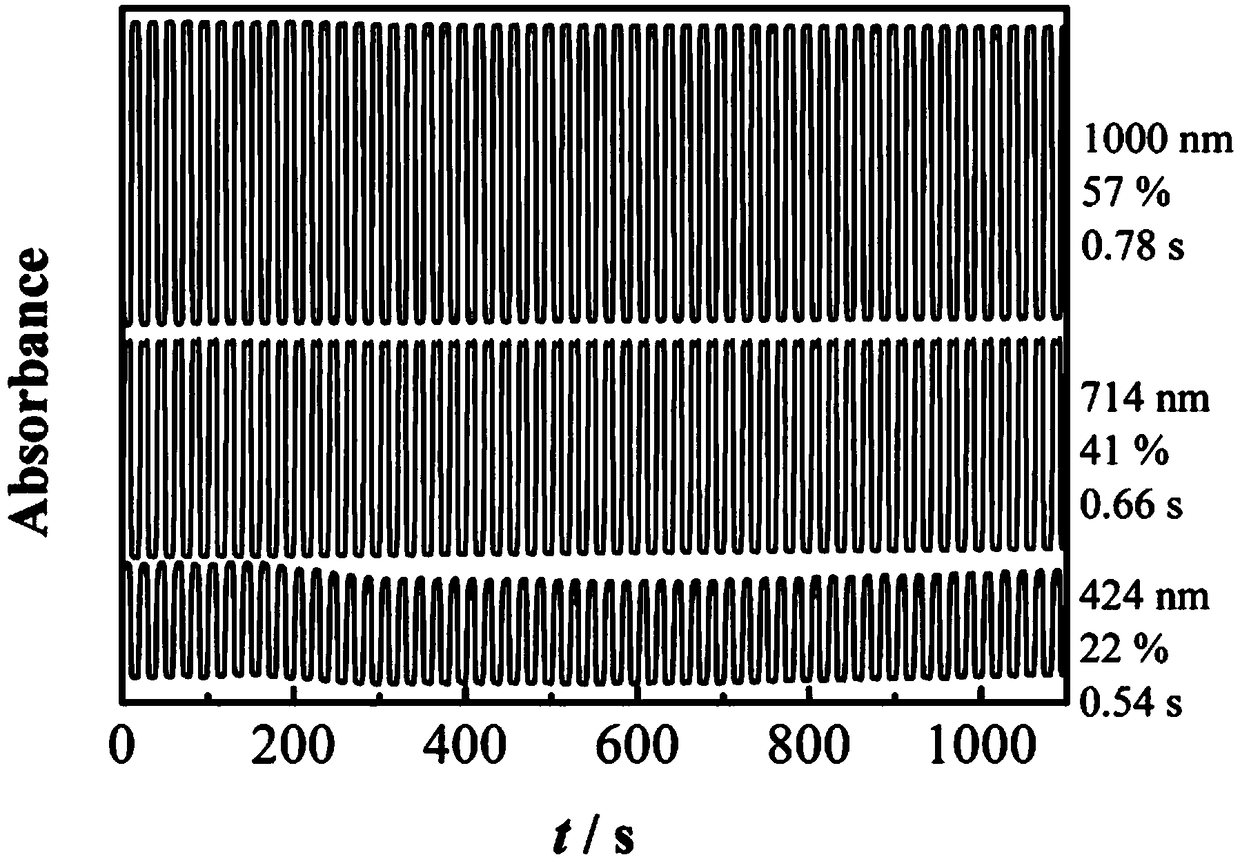
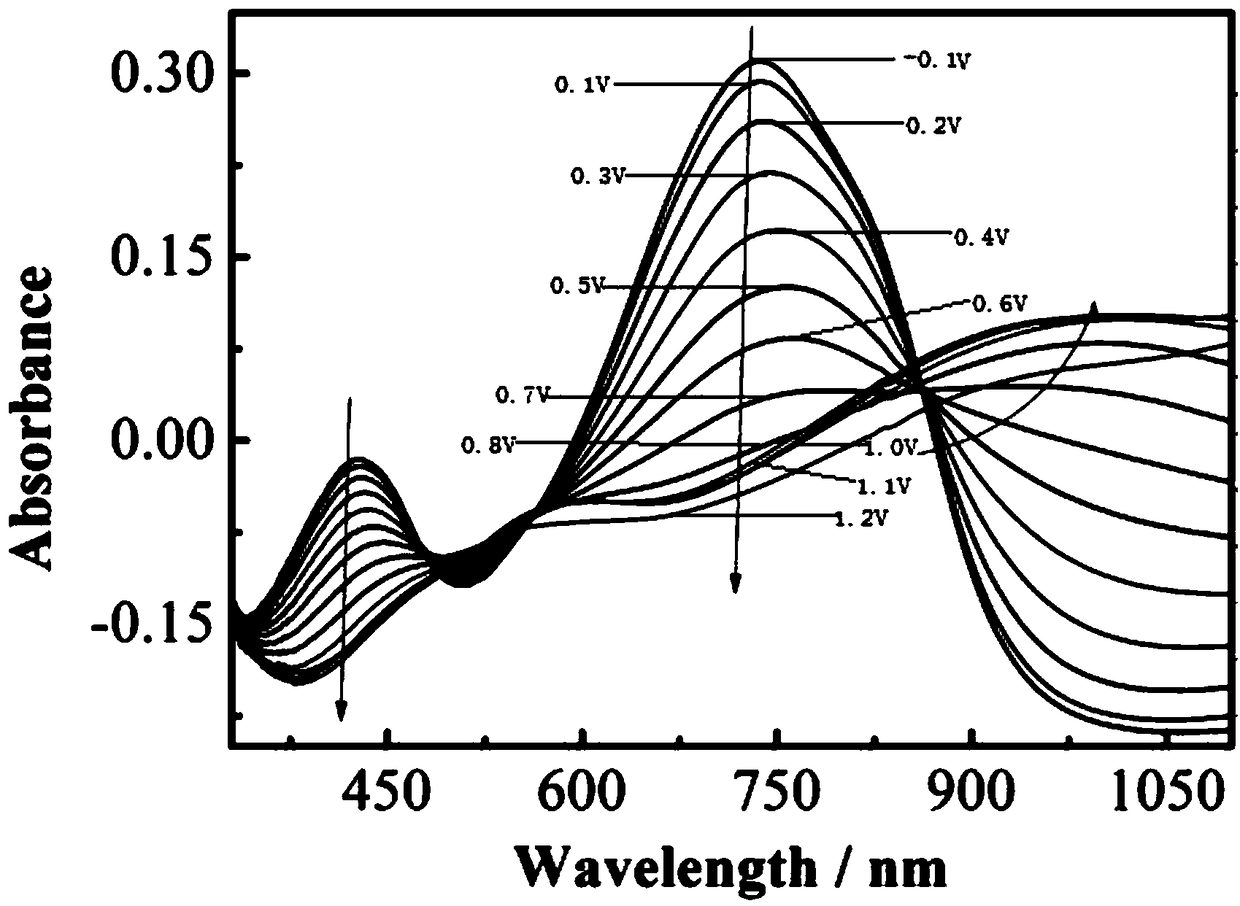
Bidiindoledione d-a-d type polymer electrochromic material and preparation method thereof
An electrochromic material, D-A-D technology, applied in the direction of color-changing fluorescent materials, chemical instruments and methods, etc., can solve the problems of increasing the difficulty of polymerization and restricting the development of polymers in the field of electrochromism, achieving a wide light absorption range and wide application range Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: the electrochemical synthesis of P (IDOH-EDOT) polymer electrochromic material
[0060] (1) Synthesis of IDOH-EDOT active precursor
[0061]
[0062] Under the condition of nitrogen protection, add a certain proportion of 6,6'-dibromo-3,3'-dihexylindoledione, 2-tinbutyl-3,4-ethane into a three-necked flask filled with toluene solvent Dioxythiophene, in catalyst Pd(PPh 3 ) 4 Under catalysis, it was condensed and refluxed at 110°C for 12 hours; after the reaction, the product was extracted three times with chloroform, dried with a small amount of anhydrous magnesium sulfate for 24 hours, and then the solvent was distilled off under reduced pressure, separated by silica gel chromatography, and purified to obtain a purple-red solid product , the yield was 80%. 1 HNMR (CDCl 3 ): δ9.14(d,J=8.47Hz,2H),7.35–7.33(dd,J 1 =8.4Hz, J2=1.6Hz, 2H), 7.22(s, 2H), 6.39(s, 2H), 4.36–4.28(m, 8H), 3.82–3.79(m, 4H), 1.65-1.55(m, 4H), 1.43–1.24(m,12H), 0.98–0.86(m,6H). ...
Embodiment 2
[0073] Embodiment 2: the electrochemical synthesis of P (IDOD-EDOT) polymer electrochromic material
[0074] (1) Synthesis of IDOD-EDOT active precursor
[0075]
[0076] Under the condition of nitrogen protection, a certain proportion of 6,6'-dibromo-3,3'-bidocosindoledione, 2-tinbutyl-3,4 -Ethylenedioxythiophene, in catalyst Pd(PPh 3 ) 4 Under catalysis, it was condensed and refluxed at 110°C for 12 hours; after the reaction, the product was extracted three times with chloroform, dried with a small amount of anhydrous magnesium sulfate for 24 hours, and then the solvent was distilled off under reduced pressure, separated by silica gel chromatography, and purified to obtain a purple-red solid product , the yield was 88%. 1 HNMR (CDCl 3 ): δ9.01(d,J=8.47Hz,2H),7.35–7.33(dd,J 1 =8.4Hz,J 2 =1.6Hz,2H),7.22(s,2H),6.39(s,2H),4.36–4.28(m,8H),3.8–3.7(m,4H),1.65-1.55(m,4H),1.43– 1.24(m,36H),0.98–0.86(m,6H).
[0077] (2) Electrochemical polymerization of P(IDOD-EDOT) polymer...
Embodiment 3
[0086] Embodiment 3: Electrochemical synthesis of P (IDOH-Th) polymer electrochromic material
[0087] (1) Synthesis of IDOH-Th active precursor
[0088]
[0089] Under nitrogen protection conditions, a certain proportion of 6,6'-dibromo-3,3'-dihexylindoledione and 2-tinbutylthiophene were added to a three-necked flask filled with toluene solvent, and the catalyst Pd( PPh 3 ) 2 Cl 2 Under catalysis, it was condensed and refluxed at 110°C for 12 hours; after the reaction, the product was extracted three times with chloroform, dried with a small amount of anhydrous magnesium sulfate for 24 hours, and then the solvent was distilled off under reduced pressure, separated by silica gel chromatography, and purified to obtain a reddish-brown solid product , the yield was 65%. 1 H-NMR (CDCl 3 ): δ9.14(d,J=8.47Hz,2H),7.35–7.33(dd,J 1 =8.4Hz,J 2 =1.6Hz,2H),7.22(s,2H),6.39(s,2H),4.36–4.28(m,8H),3.8–3.7(m,4H),1.65-1.55(m,4H),1.43– 1.24(m,12H),0.98–0.86(m,6H).(eg Figure 9 shown...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com