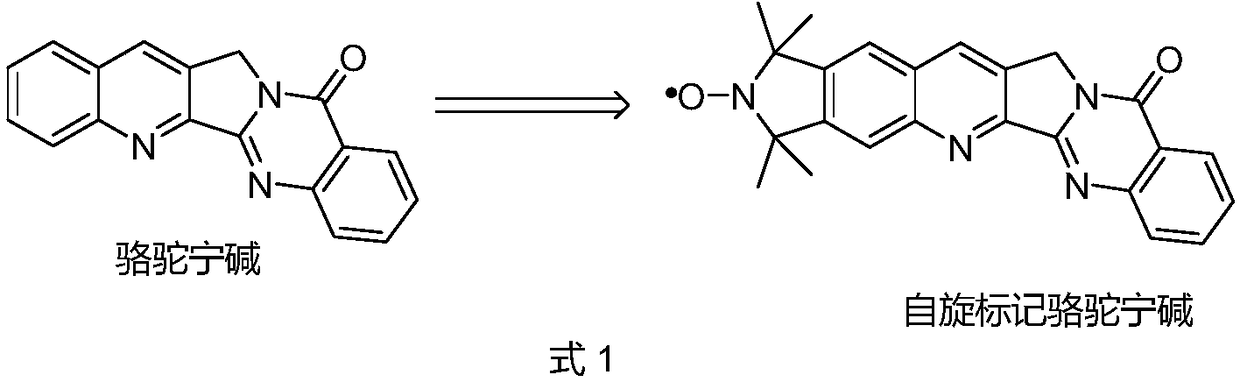
A kind of spin-labeled cameline A compound, preparation method and use thereof
A spin-labeling, camelonine technology, applied in organic chemistry, drug combination, anti-tumor drugs, etc., can solve the problems of ineffective anti-tumor activity of derivatives, low anti-tumor activity, and narrow activity spectrum, etc. Achieve the effect of novel structure, high product purity and strong inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
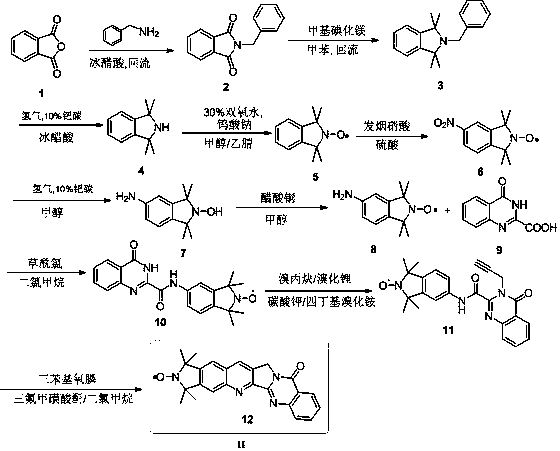
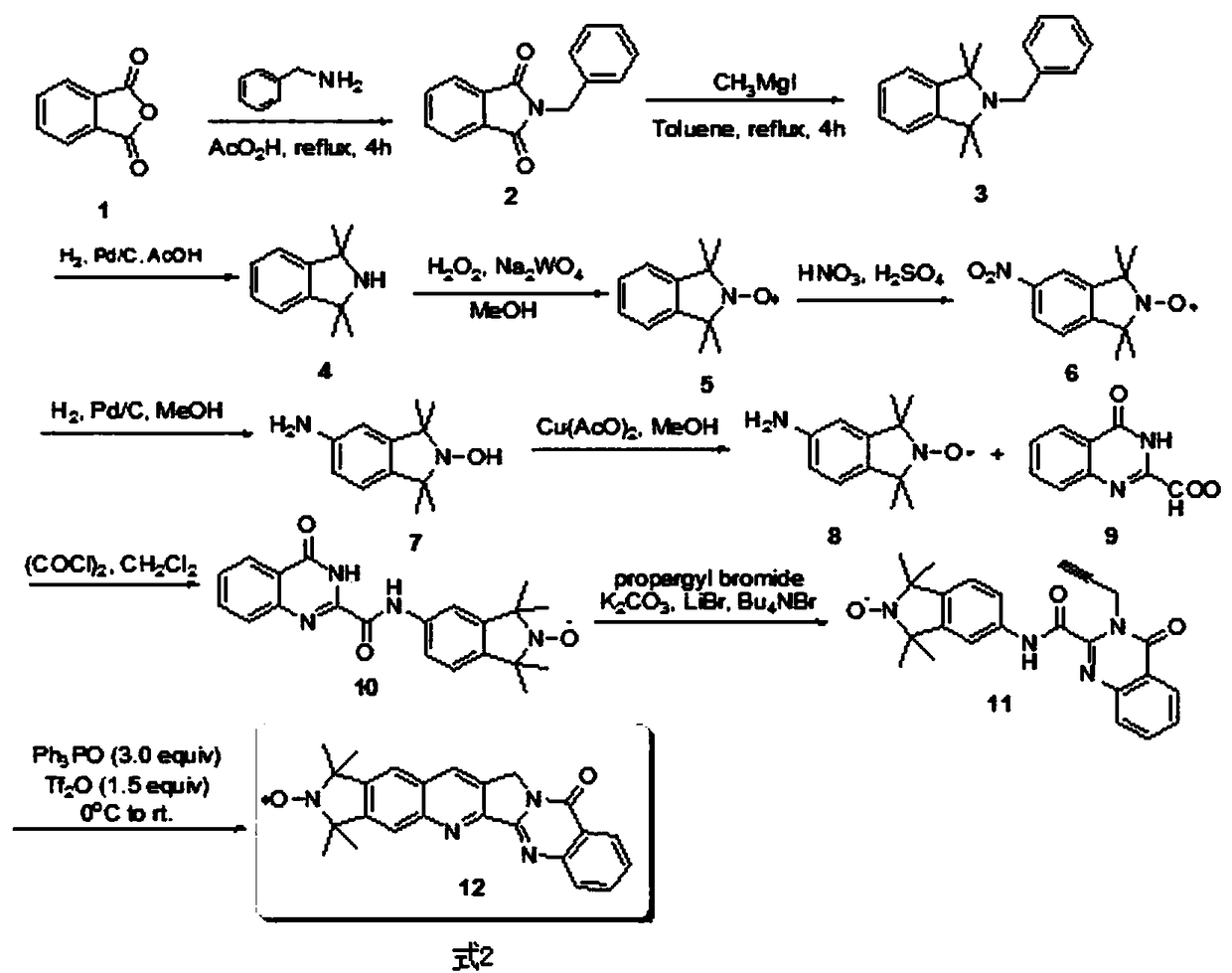
[0013] One, the preparation method of the spin-labeled cameline compound of the present invention
[0014] The following is the best preparation method of the spin-labeled camelamine compound of the present invention:
[0015] 1) Synthesis of intermediate 2: phthalic anhydride (1.69eq) was placed in an appropriate amount of glacial acetic acid, and then benzylamine (2.3eq) was slowly added therein. After the mixture was heated to reflux for 5 hours, the hot reaction solution was directly poured into ice water, and a large amount of precipitation occurred. After suction filtration, the filter cake was rinsed several times with cold water, and compound 2 was obtained after drying (refer to Chemistry-AEuropean Journal, 2009,15(47):12960-12962), see formula 6.
[0016]
[0017]2) Synthesis of Intermediate 3: First, the entire reaction device was evacuated, and replaced with argon for 3 to 5 times, then methylmagnesium iodide (6eq) was injected into a round bottom flask with a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com