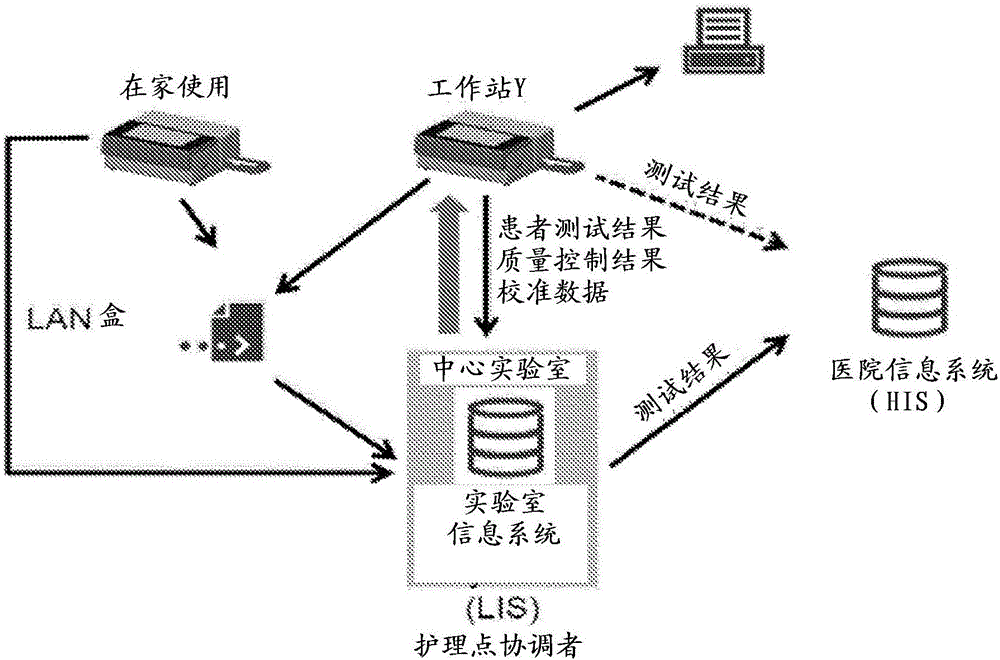
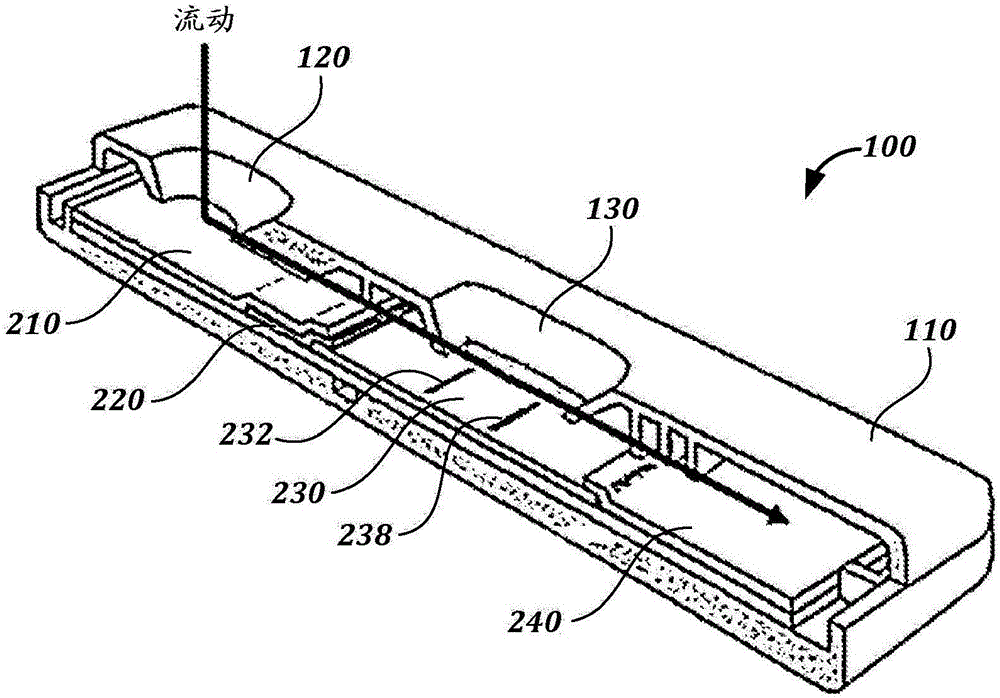
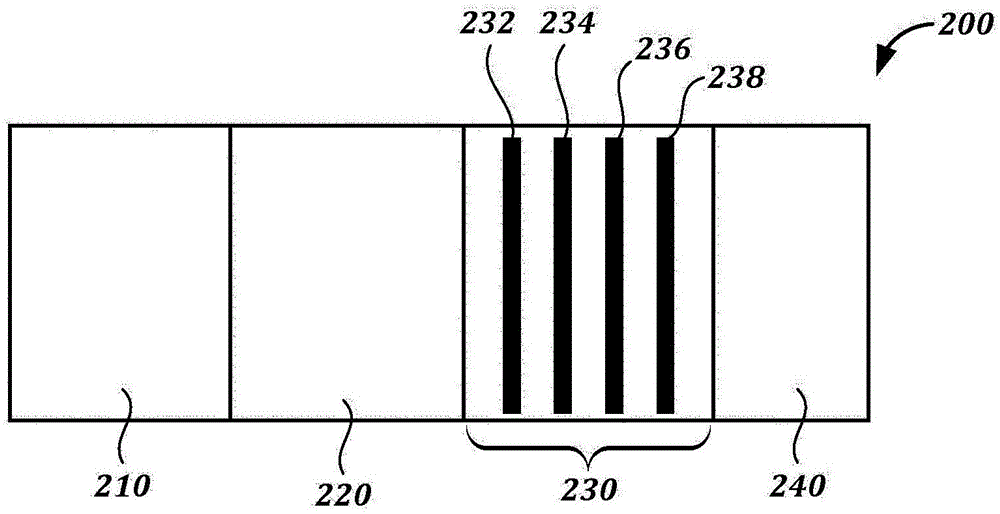
Methods, devices, and reagents for monitoring paclitaxel concentration in plasma for pharmacokinetic-guided dosing of paclitaxel
A pharmacokinetic and paclitaxel technology, which is applied to the determination of paclitaxel in liquid samples and the field of paclitaxel determination, can solve problems such as high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0187] Determination reagent
[0188] In this example, the preparation of representative detection reagents and capture reagents useful in the assay method and device of the present invention is described.
[0189] Detection reagent: antibody-colloidal gold conjugate . In short, the antibody (see Example 2) was diluted to 1 mg / mL in 0.5X PBS, and the following steps were taken: (1) Shake or vortex the gold to resuspend any precipitated gold, then add 0.5 mL of bare gold The sol is placed in 10 clean individual test tubes; (2) Each tube is marked with a pH value (or 1 to 10) from the provided pH chart; (3) The pH chart is used to add different amounts of buffer to each test tube (4) Place each test tube on a low-speed vortex mixer and add the antibody solution, then mix thoroughly (about 2 to 3 seconds), for 20nm gold, 14μL of 2mg / mL antibody or protein solution is the best; (5) Deep purple and / or black precipitates on some tubes indicate that the antibody or protein is below i...
Embodiment 2
[0194] Paclitaxel antibody
[0195] In this example, the production, processing, purification, characterization and optimization of representative paclitaxel antibodies useful in the methods and devices of the present invention are described.
[0196] Antibody production and processing. The cells were grown in CCM1 (Hyclone) containing 5-10% FBS and 1X Pen / Strep. Once the cell reaches> 1×10 6 The density of cells / mL separates the cells (1:4). The cells were then frozen and stored in 2 separate liquid nitrogen freezing tanks as a backup. Culture the cells in a roller bottle until it reaches 1×10 6 The density of cells / mL. At this time, the culture was no longer fed, and cell viability was monitored daily. Once the cell viability is reduced to <50%, remove the cells and harvest the antibody-rich medium.
[0197] Affinity purification of antibodies. Diafiltration was performed using PBS, pH 7.4, and the harvested antibody was concentrated 10-fold using a 50Kd retention membrane. ...
Embodiment 3
[0256] Representative solid phase competition assay
[0257] In this example, a representative assay demonstrating the efficacy of the solid phase competitive assay is described. This assay demonstrates the usefulness of using the anti-paclitaxel antibody described herein in this type of detection format to provide an informative signal of the presence of paclitaxel in a sample. The results indicate that the changing placement of antibodies can enhance the performance of the assay.
[0258] Paclitaxel lateral flow system. The 1.2 mg / mL BSA-Pac (test line, T) and 0.2 mg / ml goat anti-mouse antibody (control line, C) were striped onto the membrane card (High-Flow Plus HF180 membrane card, Millipore). The anti-paclitaxel antibody-colloidal gold conjugate was absorbed and dried to a binding pad (glass fiber pad, Millipore). In the assay, fetal bovine serum (FBS) spiked with paclitaxel (10 uL) (tracked by 80 μL PBS Tween) was flowed.
[0259] Tandem antibody determination. The antibo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com