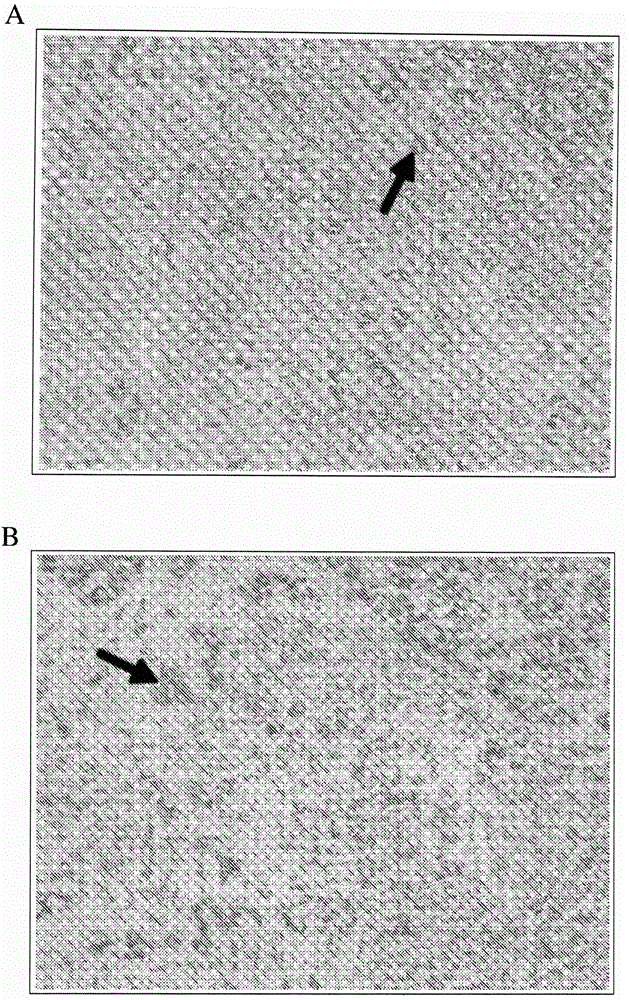
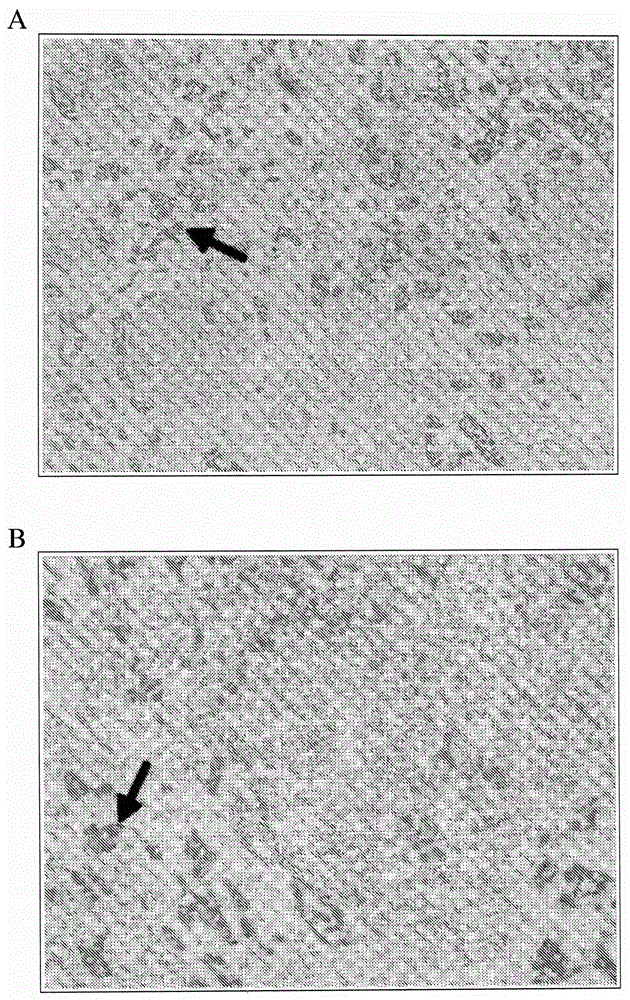
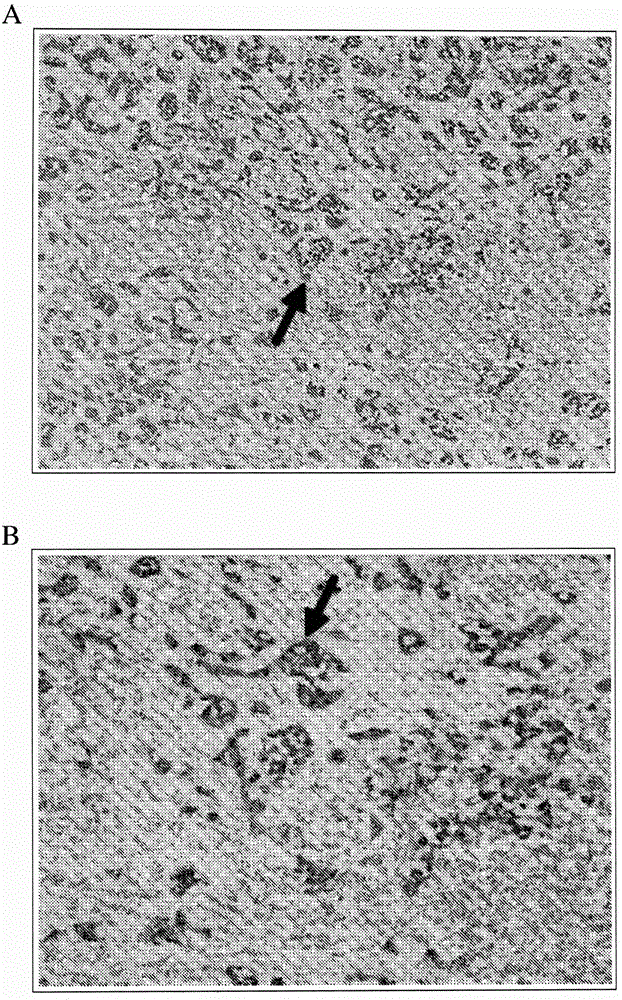
Direct immunohistochemistry assay
A technology of tissue and tissue sectioning, applied in the direction of immunoglobulin, chemical instruments and methods, biochemical equipment and methods, etc., can solve the problems of time-consuming and concealment of IHC technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] Preparation of tissue samples
[0062] Methods of preparing tissue blocks from these particulate samples have been used successfully in previous IHC studies of various prognostic factors and / or are well known to those skilled in the art.
[0063] Briefly, any whole organ or tissue can be cut into relatively small pieces and incubated in various fixatives (eg, formalin, alcohol, etc.) for varying periods of time until the tissue is "fixed." A sample can be almost any intact tissue surgically removed from the body. Samples can be cut into reasonably small piece(s) consistent with instruments routinely used in histopathology laboratories. The size of the cut pieces usually ranges from a few millimeters to a few centimeters.
[0064] In some embodiments, frozen sections can be prepared by rehydrating 50 mg of frozen "crushed" tissue in phosphate-buffered saline (PBS) at room temperature in a small plastic capsule; Centrifuge to pellet pellet; resuspend pellet in viscou...
Embodiment 1
[0303] Example 1. Protocol for Direct IHC on Frozen Tissue Slides
[0304] This example demonstrates a protocol for performing direct IHC on tissue sections obtained from frozen tissue samples.
[0305] Tissue sections were prepared as described in the Exemplary Methods below. Freshly dissected tissue blocks ( Plus, VWR).
[0306] Tissue sections were immunostained as described in the Exemplary Methods below. Tissue sections were fixed for 1 to 2 minutes with a fixative containing 75% methanol, 5% glacial acetic acid, and 20% 37% formaldehyde. Slides with tissue sections are then blocked with blocking buffer, eg, 5% skim milk or 2% BSA, for 2 minutes. After blocking, slides were washed for 10 seconds in 10 mM phosphate buffered saline (PBS) at neutral pH; slides were washed 3 times. The polymerized-HRP / antibody conjugate diluted with 1% BSA was applied to the slide to cover the entire area of the tissue section and incubated at room temperature for 3 to 5 minutes. Aft...
Embodiment 2
[0307] Example 2. Dewaxing and rehydration of tissue slides
[0308] This example demonstrates a protocol for performing direct IHC on tissue sections following deparaffinization, rehydration, and epitope retrieval of the tissue sections.
[0309] Immerse slides with tissue sections in xylene for 5 minutes. The xylene immersion was repeated two more times with clean xylene for 5 minutes each. The slides were then immersed in the following series of ethanol solutions: 100% ethanol for 3 minutes; 100% ethanol for 3 minutes; 95% ethanol for 3 minutes; and 75% ethanol for 3 minutes. Slides were then washed in tap water by immersing slides in clean tap water for 3 minutes; repeated two more times.
[0310] Following rehydration of the tissue sections, protein epitopes were exposed using trypsin. First, a 0.1% trypsin solution in 0.1% calcium chloride was prepared using distilled water. The pH of the trypsin solution was adjusted to 7.2 using 1M sodium hydroxide. The humidifi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com