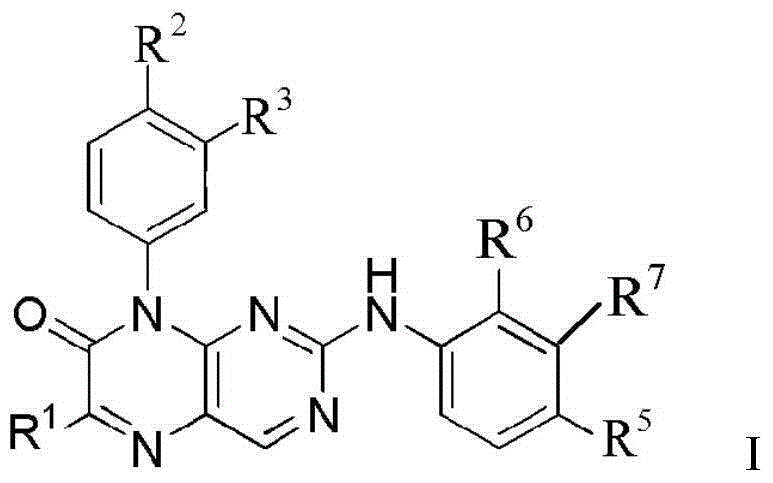
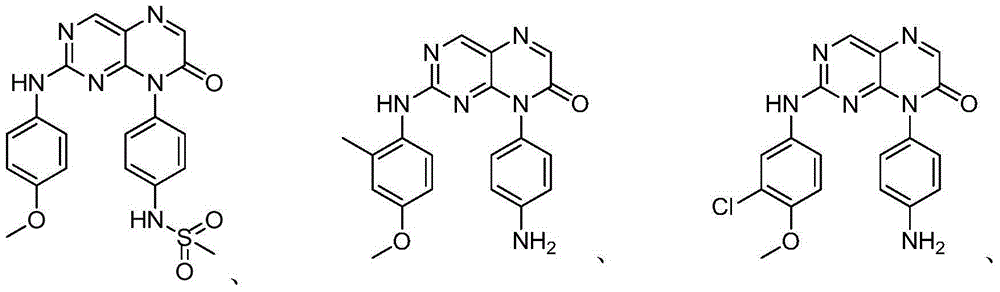
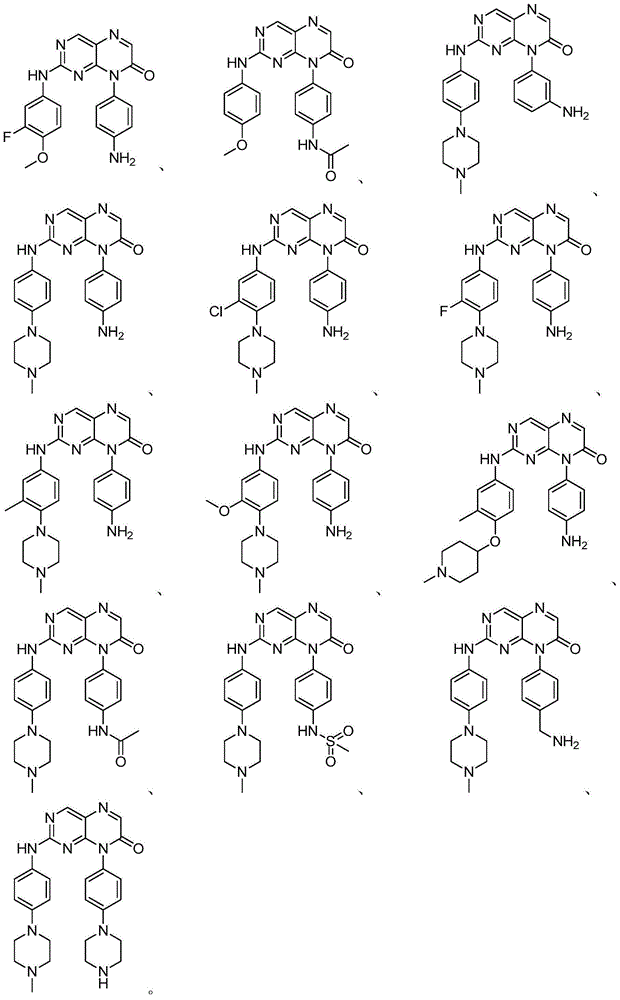
Use of pteridinone derivatives as FLT3 inhibitors
A compound, C1-C3 technology, applied in the field of treatment of FLT3-mediated diseases, can solve the problems of no practical application value and unsatisfactory clinical effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] The concrete synthetic method of above-mentioned steps a-f is as follows:
[0114] Synthesis of tert-butyl (4-(2-chloro-5-nitropyrimidine-4-amino)phenyl)carbamate (step a)
[0115]
[0116] Weigh 2,4-dichloro-5-nitropyrimidine (190mg, 0.98mmol), add 6mL 1,4-dioxane, place in a 25mL round bottom flask, stir at room temperature, take (4-aminobenzene Base) tert-butyl carbamate (200mg, 0.96mmol), N,N-diisopropylethylamine (137mg, 1.06mmol) were dissolved in 4mL 1,4-dioxane, and slowly added dropwise to the above reaction solution After the dropwise addition, the mixture was stirred at room temperature for about 1 hour, and the reaction was completely converted as monitored by TLC. The solvent was removed by rotary evaporation, and the crude product was separated by silica gel column chromatography (petroleum ether / ethyl acetate=10:1, v / v) to obtain (4-(2-chloro-5-nitropyrimidine-4-amino)phenyl ) tert-butyl carbamate orange solid 301 mg, yield 82%. 1 H NMR (400MHz, DMS...
Embodiment 2
[0179] Biological Activity Test Section
[0180] The in vitro inhibitory effect experiment of the compounds provided by the invention on FLT3 kinase activity was carried out as follows, wherein FLT3 was purchased from BPS, MLN518 and AC220 were used as reference compounds, and the reference compounds were purchased from Selleck. The two structures were as follows:
[0181]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com