Recombinant CAR gene and carrier, CAR-T cell and application thereof
A gene carrier and gene technology, applied in the field of CAR-T cells and applications, recombinant CAR genes and their vectors, which can solve the problems of T cell activation and insufficient proliferation ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Construction of embodiment 1CART-19scFv plasmid
[0046] The method for obtaining the CART-19scFv plasmid is as follows: the complete sequence of the recombinant CAR gene (SEQ ID NO: 7) is synthesized, and then ligated into the lentiviral plasmid vector pLent-EF1a by AsisI / NsiI double enzyme digestion. For the plasmid map of the constructed CART-19scFv plasmid and pLent-EF1a, see Figure 5 and Figure 6 .
Embodiment 2
[0047] Embodiment 2 T lymphocyte transfection
[0048] The constructed CART-19scFv plasmid was subjected to lentiviral packaging and purification, and then the lentiviral vector technology was used (references: Tumaini B, Lee DW, Lin T, Castiello L, et al. Simplified process for the production of anti-CD19-CAR engineered T cells.Cytotherapy.2013; 15(11):1406-1415.) Express the recombinant CAR (SEQ ID NO:7) gene in the patient's T cells, and use flow cytometry to detect the positive rate of green fluorescent protein (GFP), see results Figure 4 ,Depend on Figure 4 It can be seen that the transfection efficiency of lentivirus infection of T lymphocytes is about 30%.
Embodiment 3
[0049] Example 3 In vitro proliferative ability detection of T lymphocytes after transfection
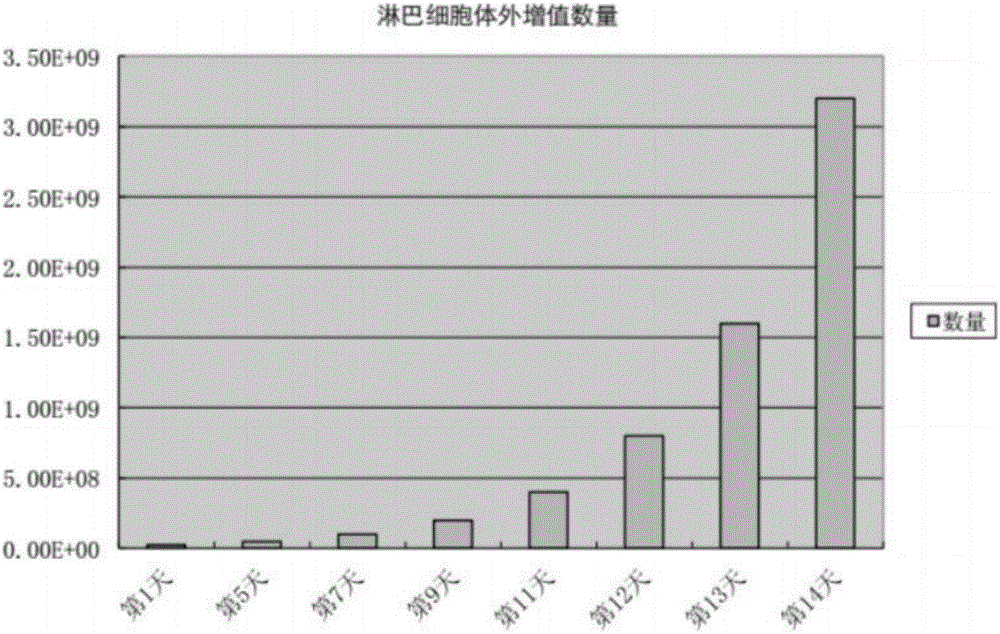
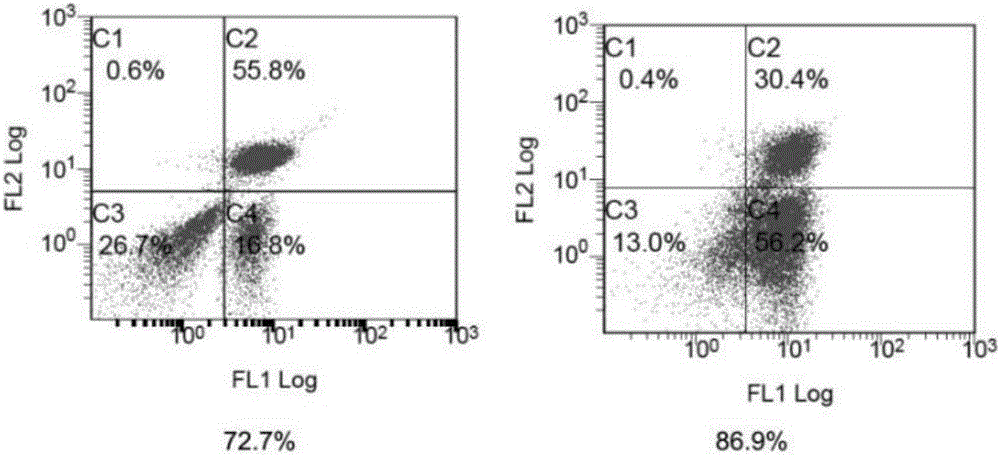
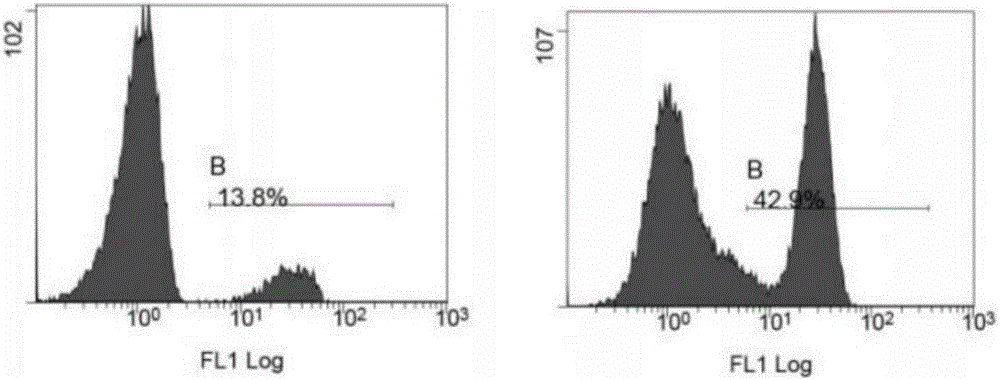
[0050] The transfected T lymphocytes (CAR-T cells) were expanded in vitro for 2 weeks, and the results are shown in figure 1 . And use flow cytometry to measure the ratio of CD3 positive T cells and CD8 positive T cells before and after expansion, the results are shown in figure 2 and image 3 . Depend on figure 2 It can be seen that the proportion of CD3 positive T cells before expansion is 55.8%+16.8%, about 72.7% in total; the proportion of CD3 positive T cells after expansion is 30.4%+56.2%, about 86.9% in total, compared with before expansion, After 2 weeks of in vitro expansion, CD3 positive T cells increased by 14.2%. Depend on image 3 It can be seen that the proportion of CD8-positive T cells before expansion was 13.8%; the proportion of CD8-positive T cells after expansion was 42.9%. Compared with before expansion, after 2 weeks of in vitro expansion, CD8-positive...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com