Application of piericidin compound Piericidin A in preparation of anti-renal cancer drugs
A technology of fenopteromycin and compounds, applied in the field of natural products, can solve problems such as weak research on mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Inhibitory activity of Piericidin A (Piericidin A) on three renal cancer cell lines
[0035] Three human renal cell lines were ordered from Shanghai Cell Resource Center, Chinese Academy of Sciences: 786-O human renal cell line (Cat#TCHu186); ACHN human renal cell line (Cat# TCHu199); OS-RC-2 human renal cell line strain (Cat# TCHu40).
[0036] The inhibitory activity of renal cancer cells was tested by CCK-8 assay. Collect the cells in the logarithmic growth phase, count them, resuspend the cells with complete medium, adjust the cell concentration to an appropriate concentration (determined according to the results of the cell density optimization test), inoculate a 96-well plate, and add 100 μl of cell suspension to each well. Cells were incubated for 24 hours at 37°C, 100% relative humidity, 5% CO2 incubator. Dilute the compound to be tested to an appropriate concentration with complete medium, and add 25 μl / well to the cells. For ACHN cells, the final ...
Embodiment 2
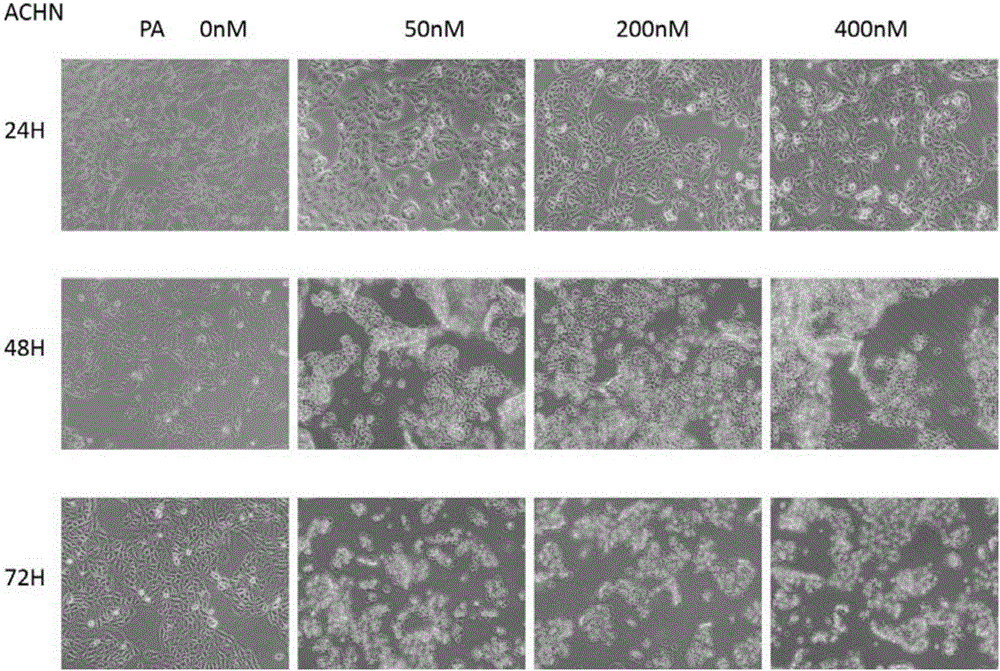
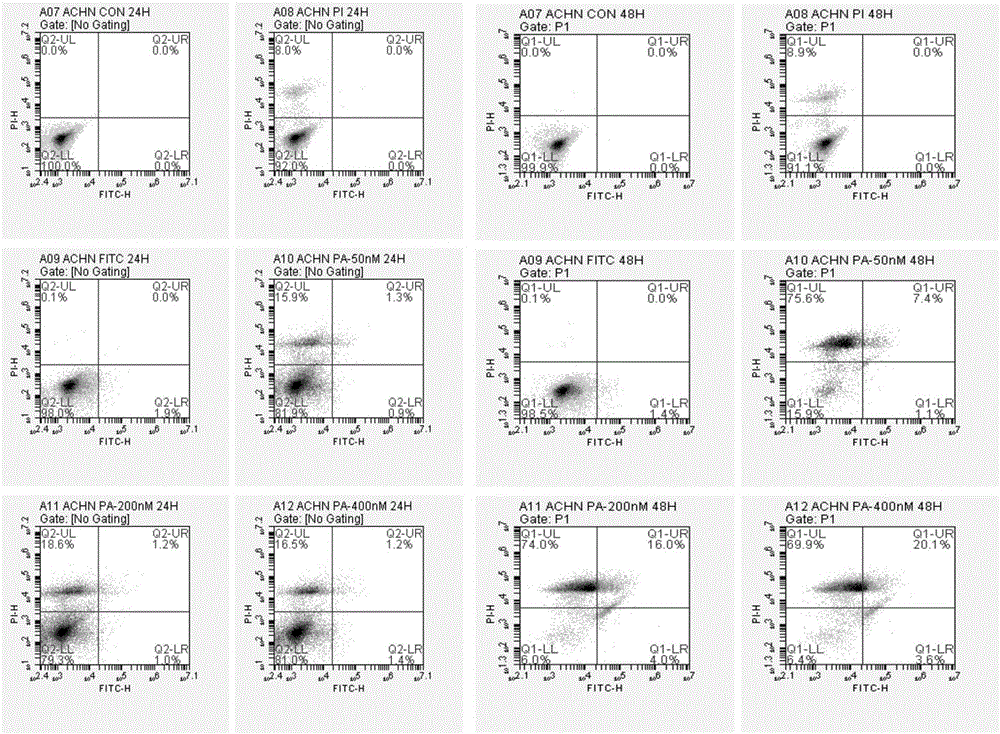
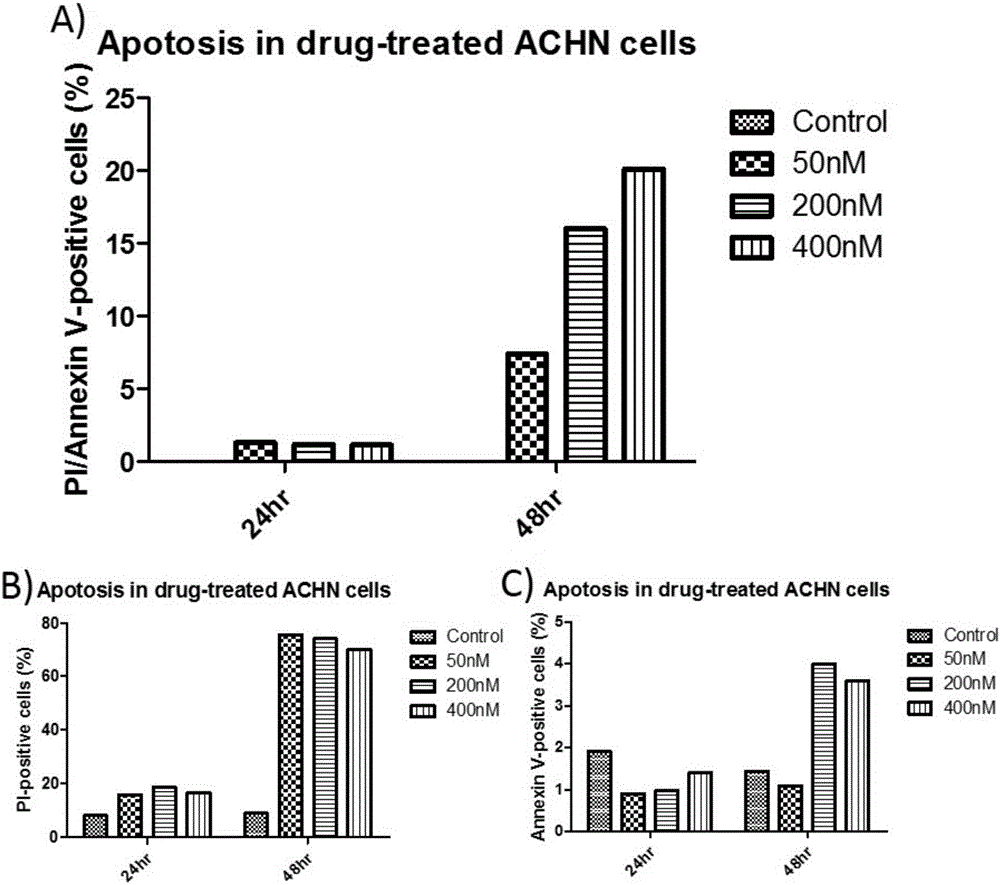
[0048] Example 2: Effects of Piericidin A on the Apoptosis of Three Renal Cancer Cell Lines ACHN Cells
[0049]Take the ACHN cells in the logarithmic growth phase and adjust the cell concentration to 2×10 6 / ml, inoculated in 50mL culture flasks, divided into three batches, each batch was divided into four groups, group 1 was blank control, group 2, 3, and 4 were added Piericidin A respectively, so that the final concentration was 50, 200 and 400nM. After the cells adhered to the wall, the culture medium was replaced and drugs were added. Two batches were cultured for 24 hours and 48 hours respectively, and the cells were digested and collected after light microscope observation and photography. The third batch was cultured until 72 hours for light microscope observation and photography. The collected cells were washed with pre-cooled PBS at 4°C, centrifuged at 1000r / min, 2×5min. After the cells were resuspended in the buffer, 100 μL of the cell suspension was taken in a flow...
Embodiment 3
[0052] Embodiment 3: Piericidin A suppresses the experiment of Bcl-2 protein expression
[0053] ACHN cells in logarithmic growth phase were taken, and Piericidin A was added to make the final concentration of 0, 10, 50 and 200nM, and the effect was 48h. ① Extraction of total protein: After washing twice with PBS, add 200 μL of protein lysate RIPA and 5 μL / mL of PMSF to each sample, place in an ice box for 40 min; centrifuge at 15 000 r / min, 4°C for 20 min; take the supernatant That is the required protein extract, and aliquoted in 100 μL tubes. After detecting the protein concentration with a micro-ultraviolet spectrometer, store it at -80°C for later use. ② Polyacrylamide gel electrophoresis (SDS-PAGE electrophoresis): Take 100 μL of protein sample and add 4×loading buffer, denature at 100°C for 10 minutes; load the sample, and run electrophoresis for about 1.5 hours. ③Membrane transfer: The protein was transferred to nitrocellulose membrane (PVDF) by semi-dry electrotrans...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com