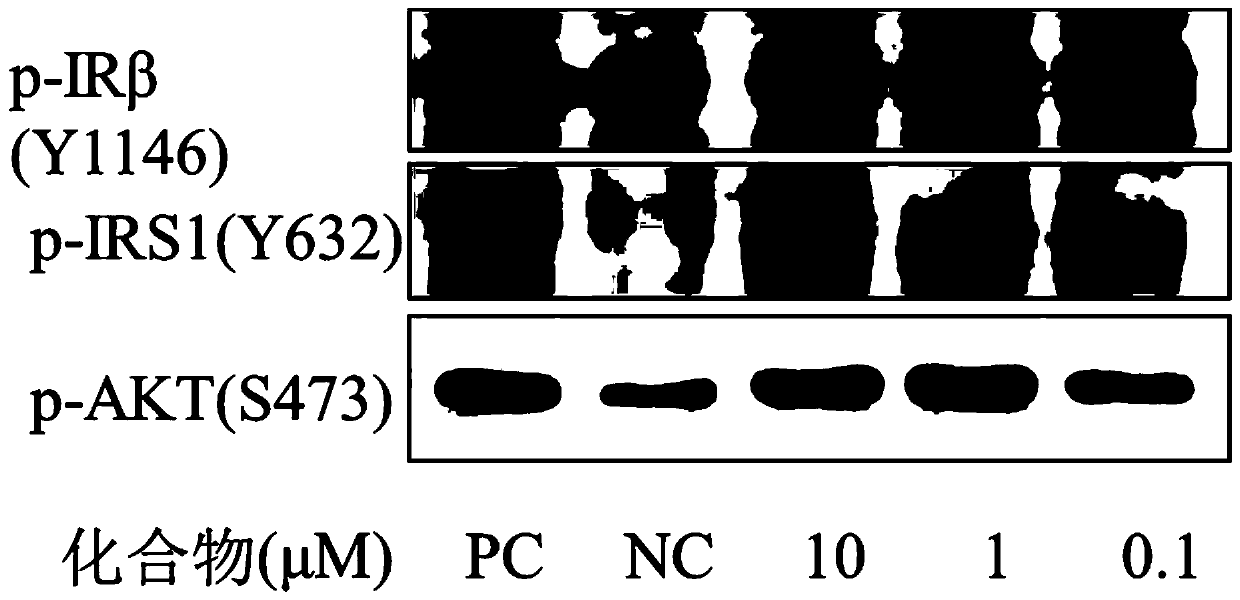
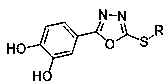
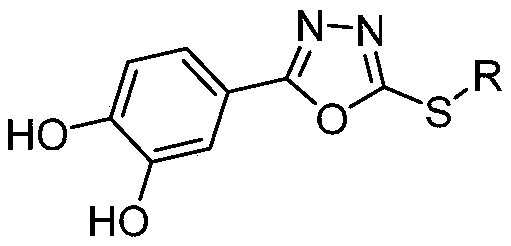
Oxadiazole compounds and their application in the preparation of drugs for preventing and/or treating type 2 diabetes
A technology of type 2 diabetes and oxadiazoles, which is applied in the field of oxadiazole compounds and their application in the preparation of drugs for the prevention and/or treatment of type 2 diabetes, and can solve the problems of low cell permeability and bioavailability, Problems such as high hydrophilicity and electronegativity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Synthesis and structural identification of 4-(5-(benzylthio)-1,3,4-oxadiazole-2-substituted)benzene-1,2-hydroxyl
[0020]
[0021] 15.4 g (0.1 mol) of 3,4-dihydroxybenzoic acid and 200 mL of absolute ethanol were added to a 500 mL flask, and 5 mL of 98% concentrated sulfuric acid was slowly added dropwise with constant stirring. The mixture was heated to reflux, the reaction was followed by TLC, 80% of the volume of the solvent was evaporated under reduced pressure, diluted with 30 mL of ethyl acetate and 25 mL of water, the aqueous layer was extracted with 300 mL of ethyl acetate, the organic layers were combined, and saturated NaHCO was added. 3 Aqueous solution, washed with saturated brine, anhydrous Na 2 SO 4 Dry, filter, and remove the solution under reduced pressure to obtain 15.8 g of ethyl 3,4-dihydroxybenzoate with a yield of 86.8%.
[0022] Take 15.8g of the solid ethyl 3,4-dihydroxybenzoate from the previous step, add it to 100mL of absolute e...
Embodiment 2
[0030] Example 2: Synthesis and Structural Identification of p-Tolyl-2-((5-(3,4-Dihydroxyphenyl)-1,3,4-oxadiazole-2-substituted)mercapto)acetate
[0031]
[0032] 15.4 g (0.1 mol) of 3,4-dihydroxybenzoic acid and 200 mL of absolute ethanol were added to a 500 mL flask, and 5 mL of 98% concentrated sulfuric acid was slowly added dropwise with constant stirring. The mixture was heated to reflux, the reaction was followed by TLC, 80% of the solvent was evaporated under reduced pressure, diluted with 30 mL of ethyl acetate and 25 mL of water, the aqueous layer was extracted with ethyl acetate, the organic layers were combined and washed with saturated NaHCO 3 Aqueous solution, washed with saturated brine, anhydrous Na 2 SO 4 Dry, filter, and remove the solution under reduced pressure to obtain 15.8 g of ethyl 3,4-dihydroxybenzoate with a yield of 86.8%.
[0033]Take 15.8g of the solid ethyl 3,4-dihydroxybenzoate from the previous step, add it to 100mL of absolute ethanol, hea...
Embodiment 3
[0041] Example 3: Synthesis and structure of 3-ethoxybenzene 2-((5-(3,4-dihydroxyphenyl)-1,3,4-oxadiazole-2-substituted)mercapto)acetate identification
[0042]
[0043] 15.4 g (0.1 mol) of 3,4-dihydroxybenzoic acid and 200 mL of absolute ethanol were added to a 500 mL flask, and 5 mL of 98% concentrated sulfuric acid was slowly added dropwise with constant stirring. The mixture was heated to reflux, the reaction was followed by TLC, 80% of the solvent was evaporated under reduced pressure, diluted with 30 mL of ethyl acetate and 25 mL of water, the aqueous layer was extracted with 300 mL of ethyl acetate, the organic layers were combined and washed with saturated NaHCO 3 Aqueous solution, washed with saturated brine, anhydrous Na 2 SO 4 Dry, filter, and remove the solution under reduced pressure to obtain 15.8 g of ethyl 3,4-dihydroxybenzoate with a yield of 86.8%.
[0044] Take 15.8g of the solid ethyl 3,4-dihydroxybenzoate from the previous step, add it to 100mL of ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com