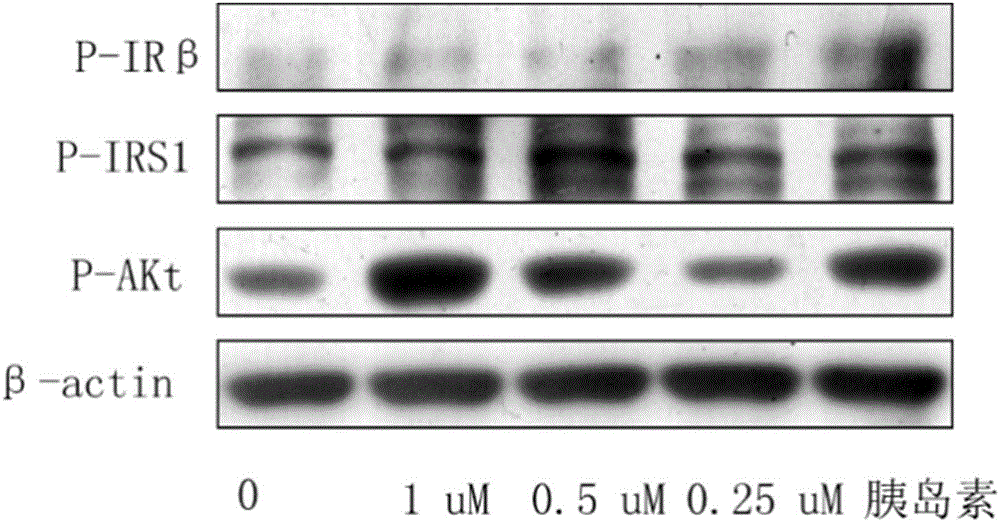
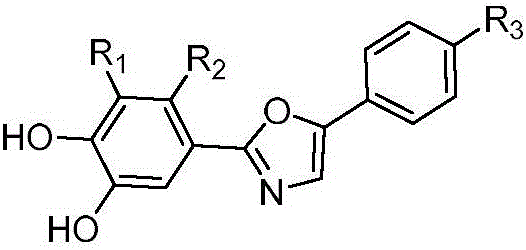
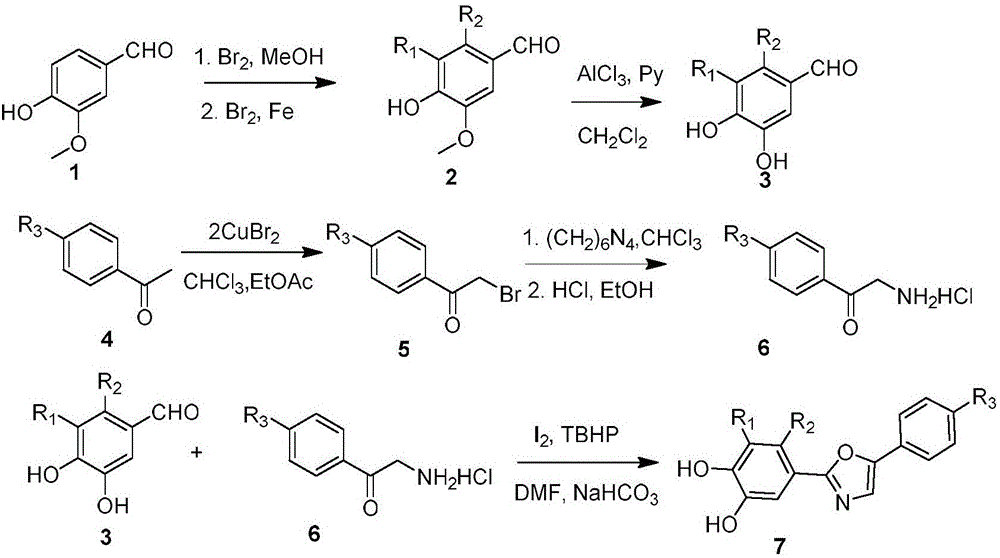
Bromophenol-oxazole compound and its use in drug for treatment on diabetes mellitus type 2
A technology for type 2 diabetes and oxazoles, which is applied to bromophenol-oxazole compounds and their application fields in medicines for the treatment of type 2 diabetes, and can solve the problems of low cell permeability and bioavailability, high hydrophilicity and electronegativity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Synthesis and structural identification of 4-(5-(4-bromophenyl)oxazole-2-substituted)benzene-1,2-dihydroxy
[0021]
[0022] Add 15.2g of 4-hydroxy-3-methoxy-benzaldehyde into 50mL of dichloromethane, stir evenly, carefully add 20g of AlCl 3 . In an ice bath, slowly add 32 mL of pyridine in 10 mL of CH 2 Cl 2 solution. After dropping, reflux for 24 hours. Cool, add concentrated hydrochloric acid to adjust pH<3, separate the aqueous phase, extract with ethyl acetate, dry, and evaporate to dryness. The solid was recrystallized from water to obtain 3,4-dihydroxybenzaldehyde as a pale yellow solid with a melting point of 153-154°C.
[0023] Take 0.5mol of 1-(4-bromophenyl)ethanone and add 150mL of ethyl acetate and chloroform mixed solution, add 22.3g of CuBr under stirring 2 . Reflux for 2 hours, filter while hot, and wash with chloroform. The mixed solution was washed with water, dried, and evaporated to dryness to obtain a brown solid, which was 2-b...
Embodiment 2
[0026] Example 2: Synthesis and structure identification of 3,4-dibromo-5-(5-(4-phenoxyphenyl)oxazole-2-substituted)benzene-1,2-hydroxy
[0027]
[0028] Add 15.2g of 4-hydroxy-3-methoxy-benzaldehyde into 100mL of dichloromethane, and stir evenly. Under stirring at room temperature, a solution of 5.6 mL of bromine in 10 mL of methanol was added dropwise. React at room temperature for 2 hours, cool to 0°C, and add 50 mL of ice water dropwise. A precipitate was washed out, filtered, washed with ice water, and dried to obtain 21 g of white crystals. The resulting solid was added to 100 mL of acetic acid, and a solution of 5.6 mL of bromine in 15 mL of acetic acid was added dropwise. Add 0.1 g of reduced iron powder, and reflux for 2 hours. After cooling, filter, and recrystallize the solid from ethyl acetate to obtain 9.6 g of white needle crystals, which are 2,3-dibromo-4-hydroxy-5-methoxy-benzaldehyde. Add the resulting 2,3-dibromo-4-hydroxy-5-methoxy-benzaldehyde into 5...
Embodiment 3
[0032] Example 3: Synthesis and structural identification of 3-bromo-5-(5-(4-(4-chlorophenylthio)phenyl)oxazole-2-substituted)benzene-1,2-dihydroxy
[0033]
[0034] Add 15.2g of 4-hydroxy-3-methoxy-benzaldehyde into 100mL of dichloromethane, and stir evenly. Under stirring at room temperature, a solution of 5.6 mL of bromine in 10 mL of methanol was added dropwise. React at room temperature for 2 hours, cool to 0°C, and add 50 mL of ice water dropwise. A precipitate was washed out, filtered, washed with ice water, and dried to obtain 21 g of white crystals, which were 2-bromo-4-hydroxy-5-methoxy-benzaldehyde. Add the resulting 2-bromo-4-hydroxy-5-methoxy-benzaldehyde into 50 mL of dichloromethane, stir well, and carefully add 20 g of AlCl 3 . In an ice bath, slowly add 32 mL of pyridine in 10 mL of CH 2 Cl 2 solution. After dropping, reflux for 24 hours. Cool, add concentrated hydrochloric acid to adjust pH<3, separate the aqueous phase, extract with ethyl acetate, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com