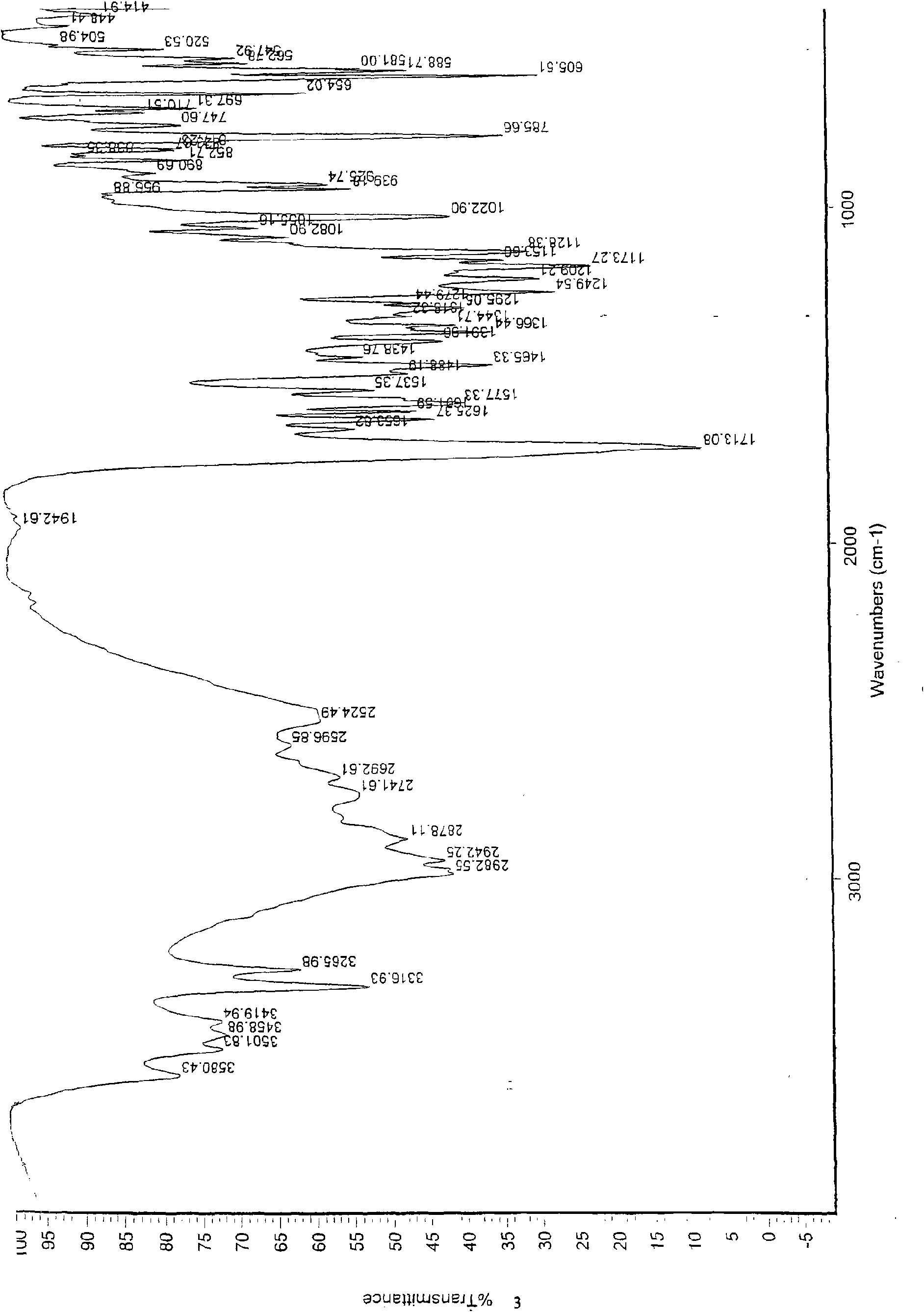
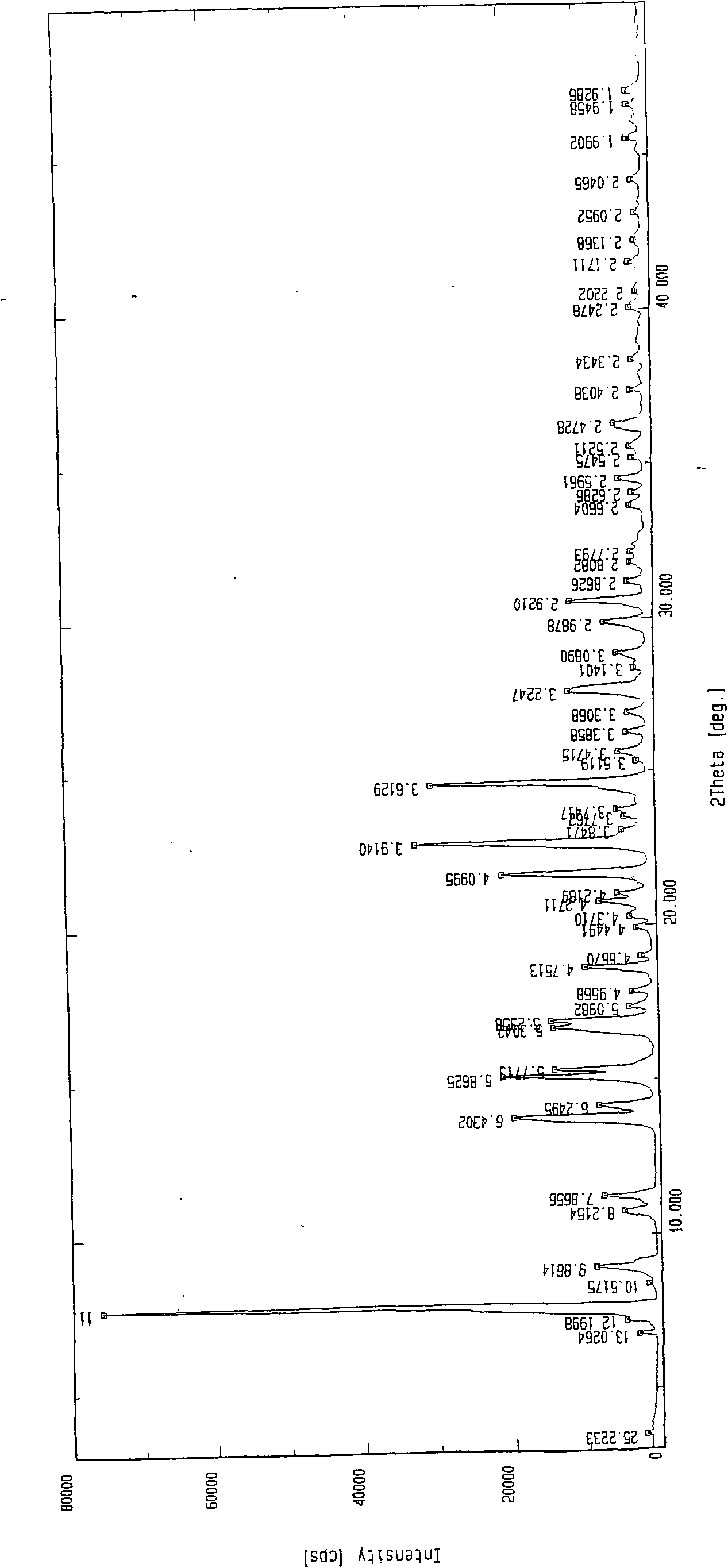
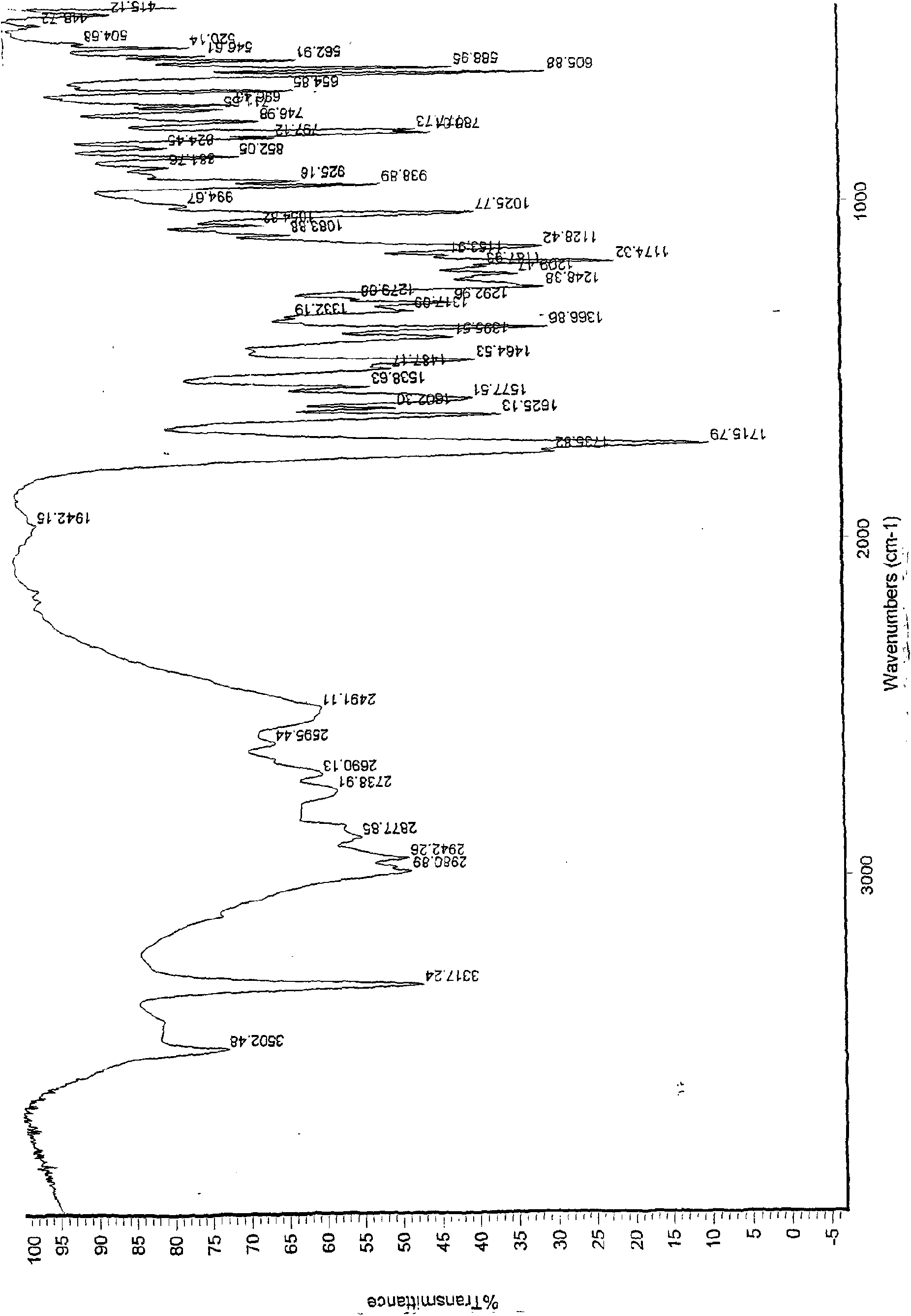
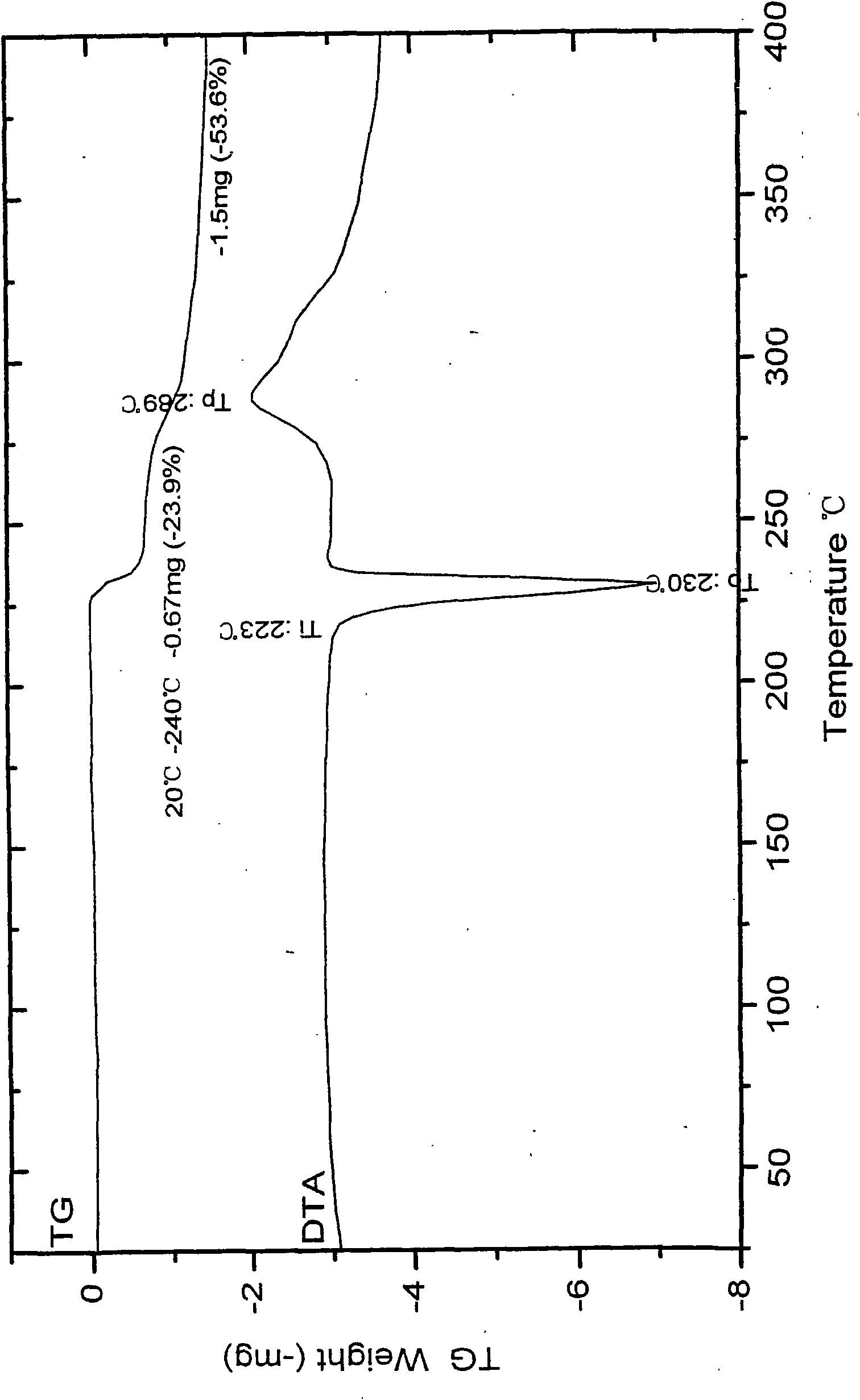
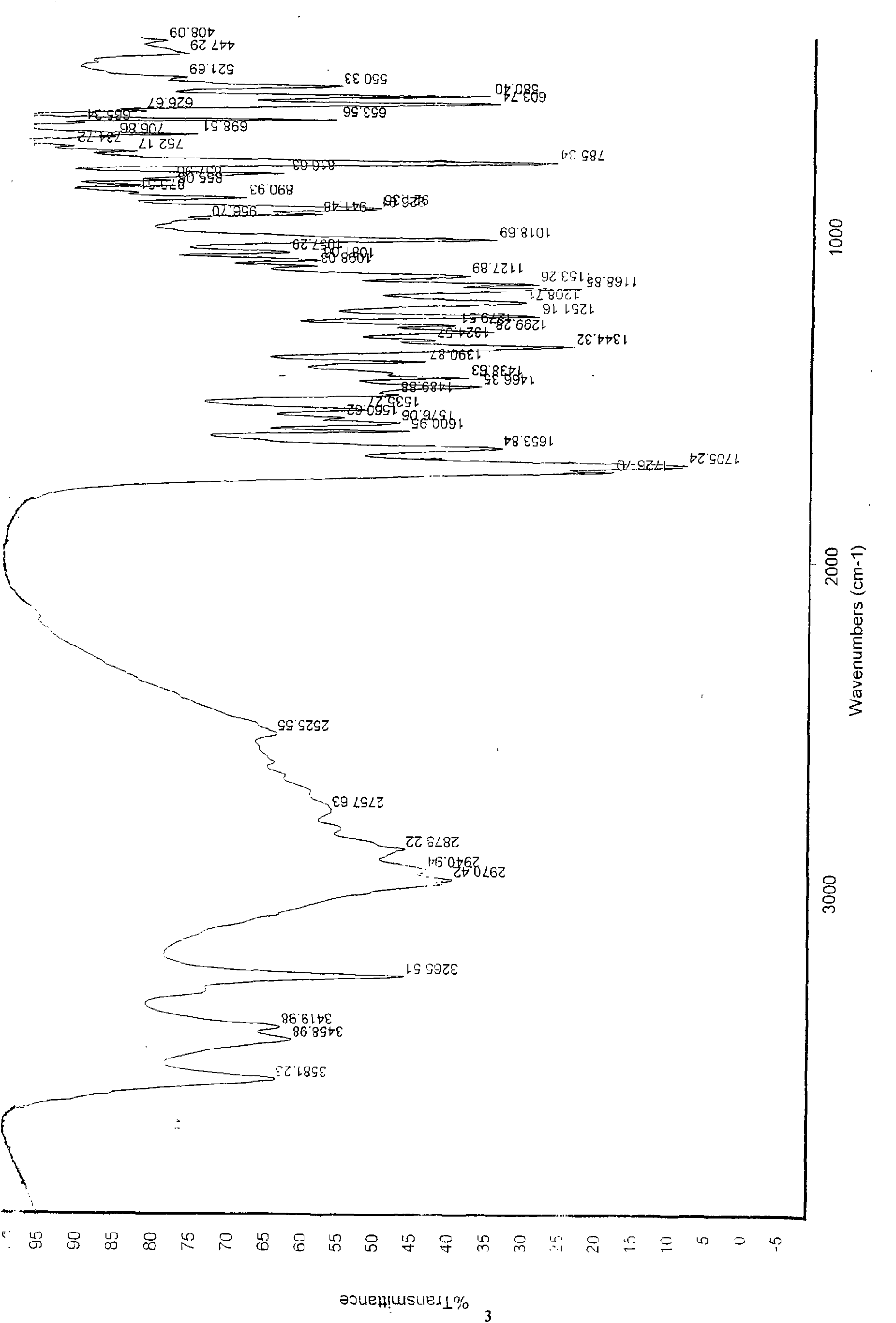
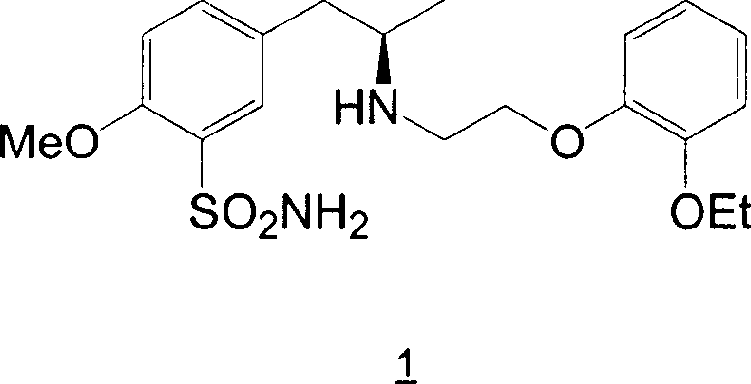
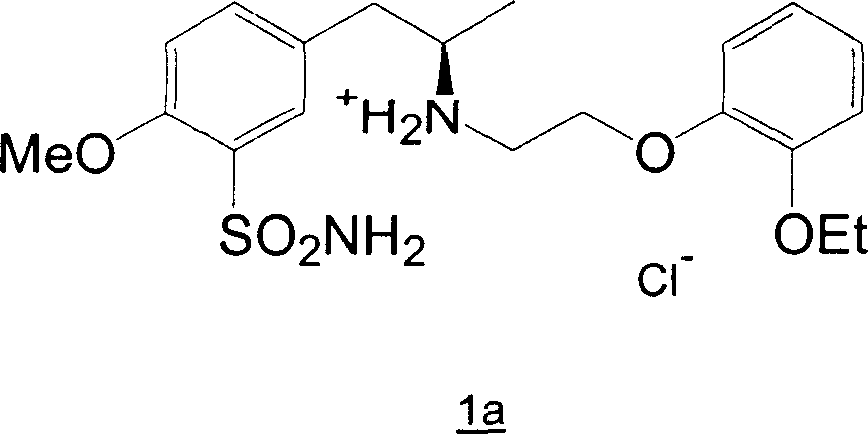
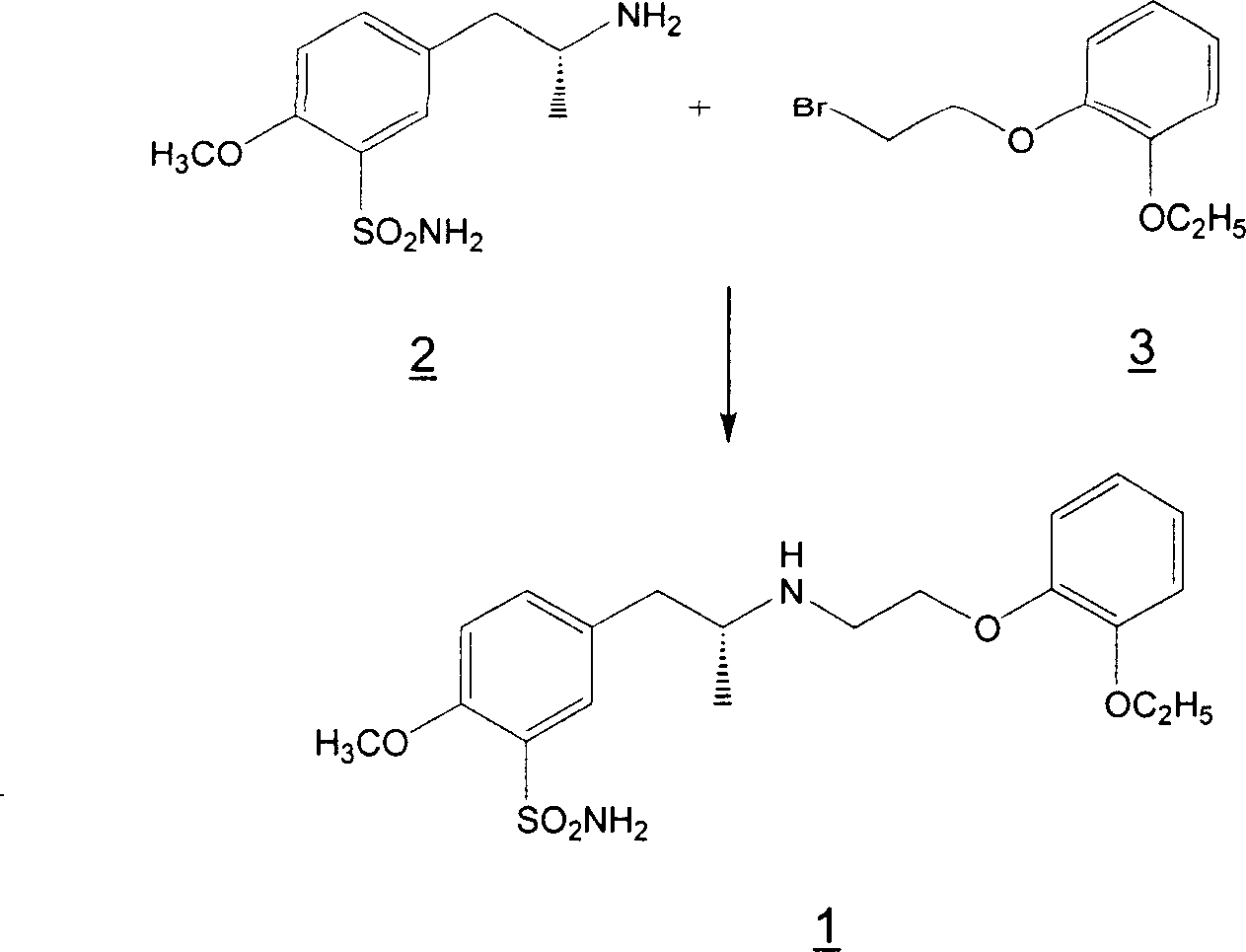
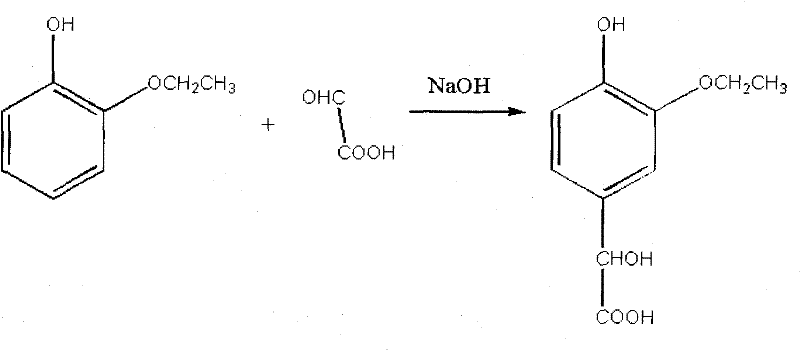
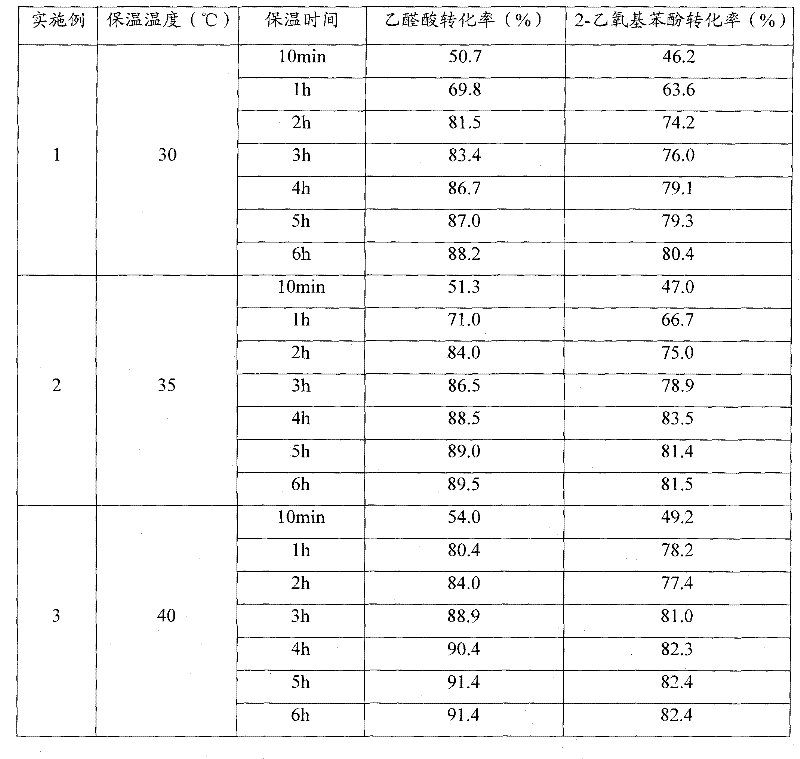
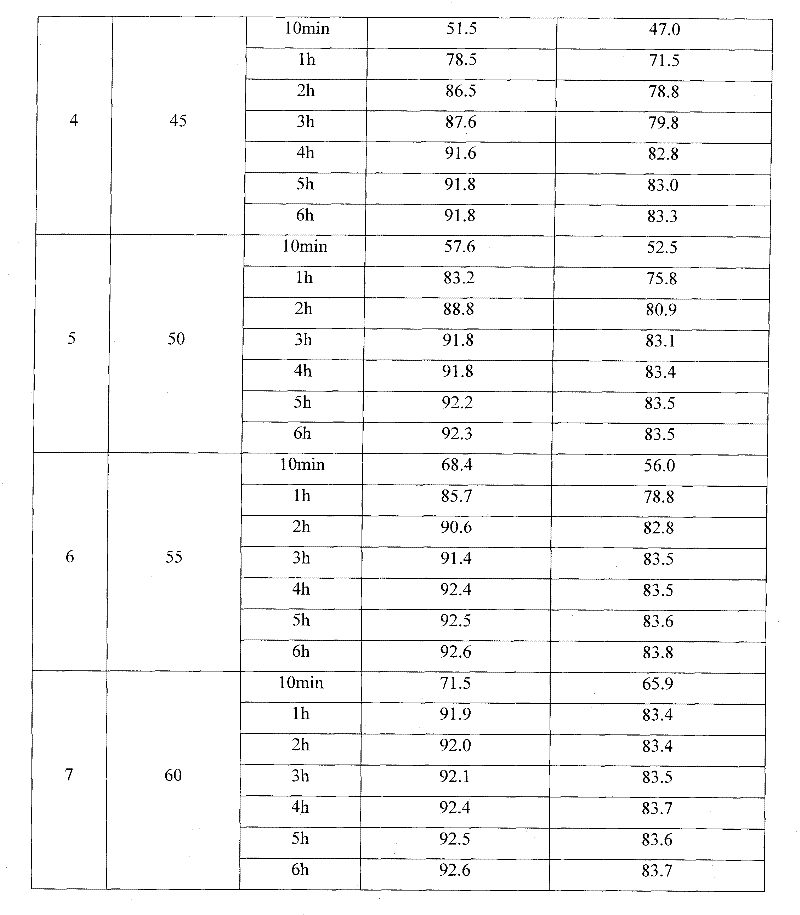
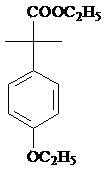
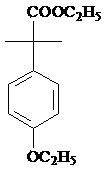
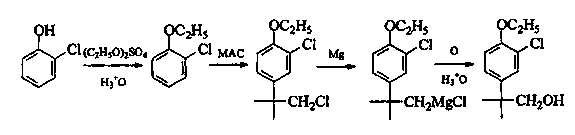
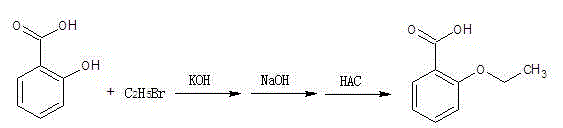
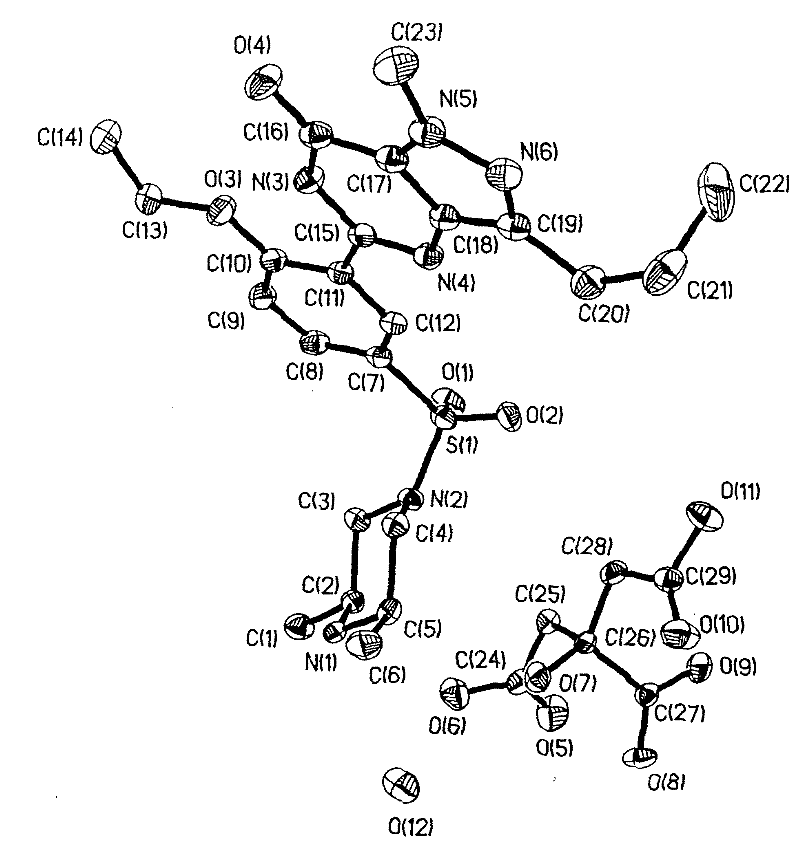
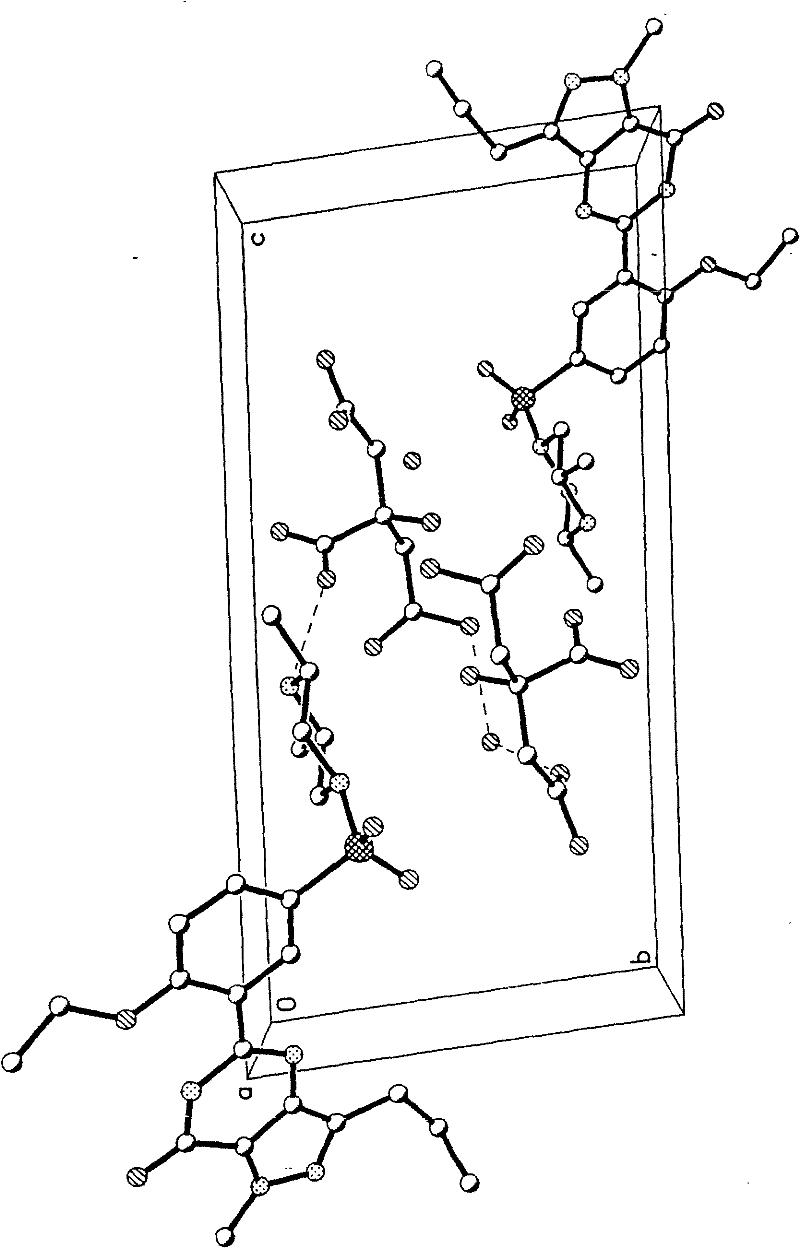
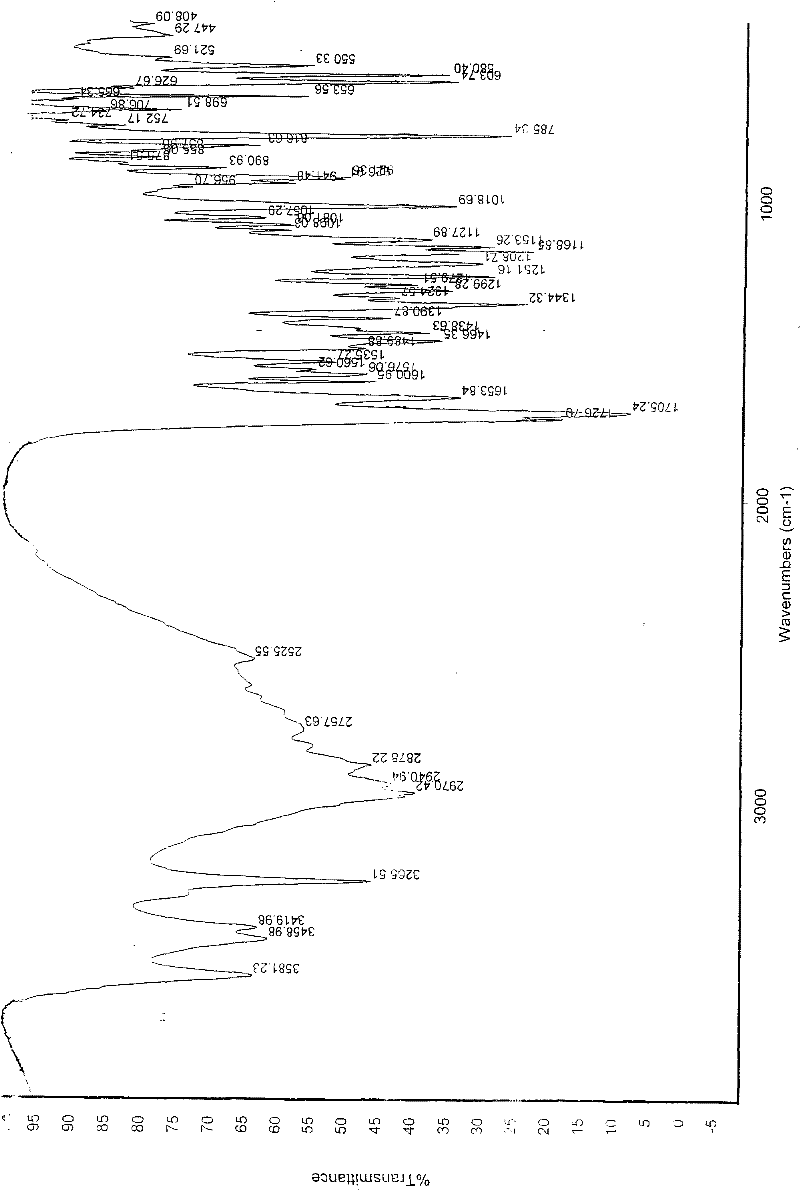
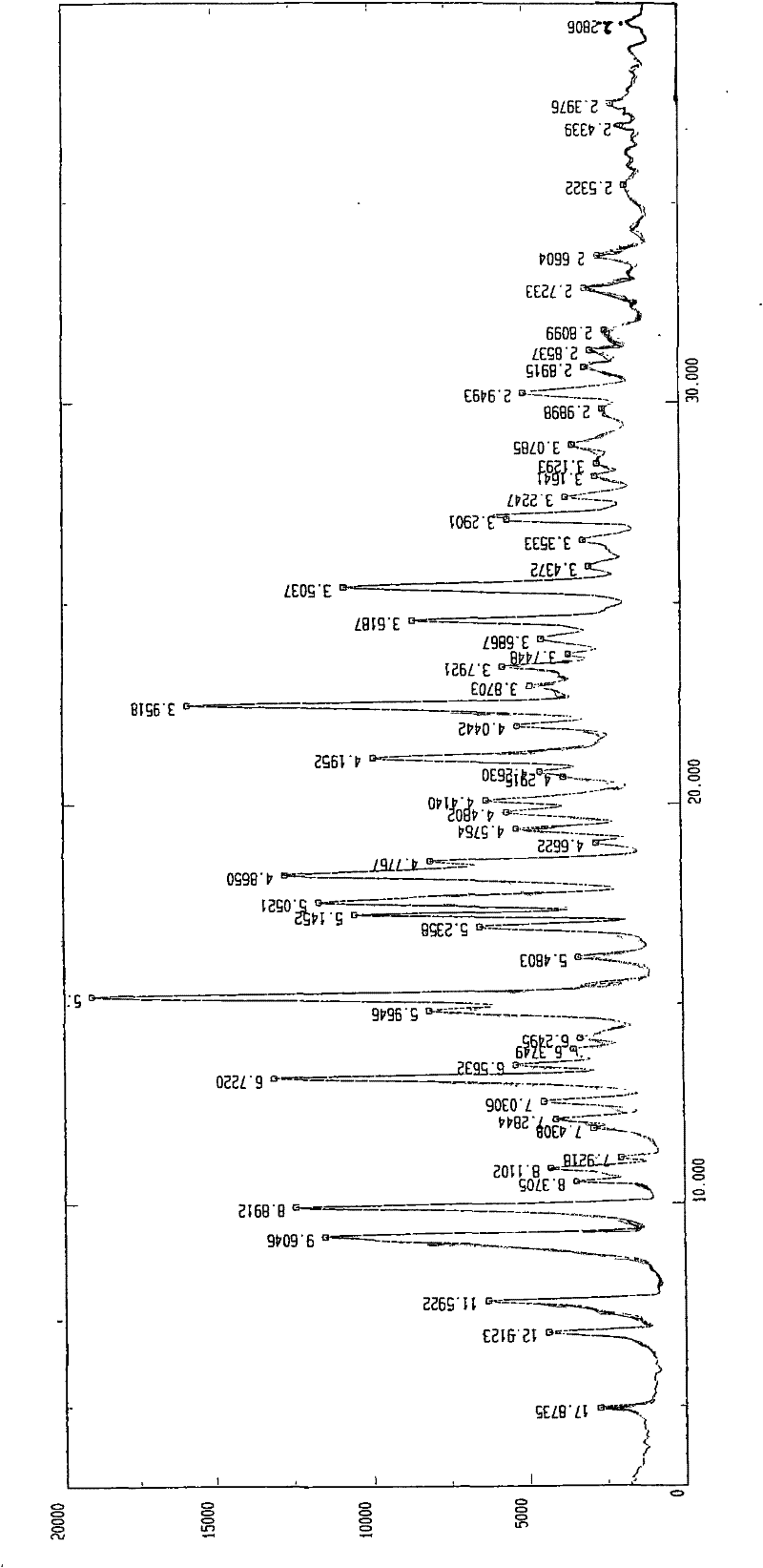
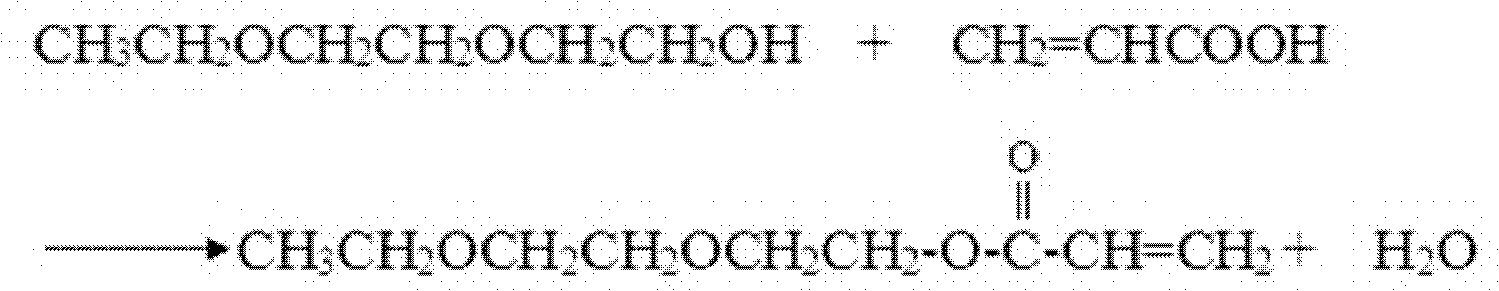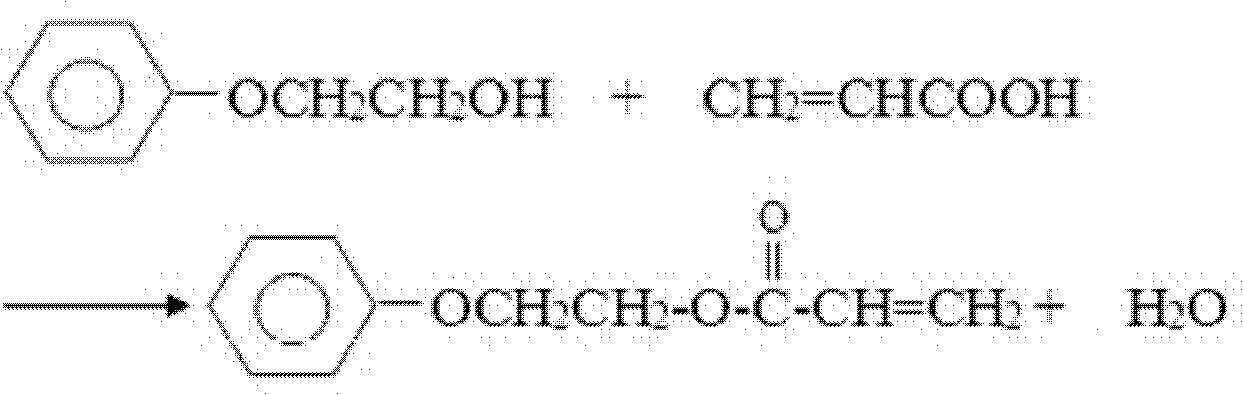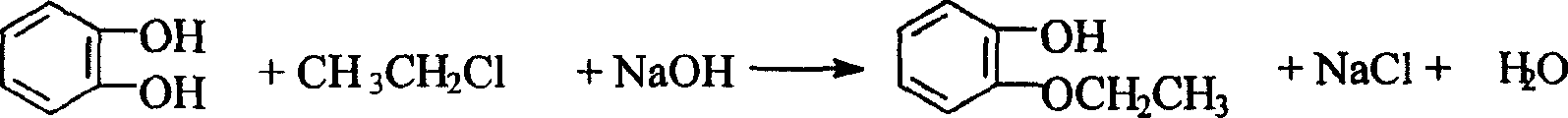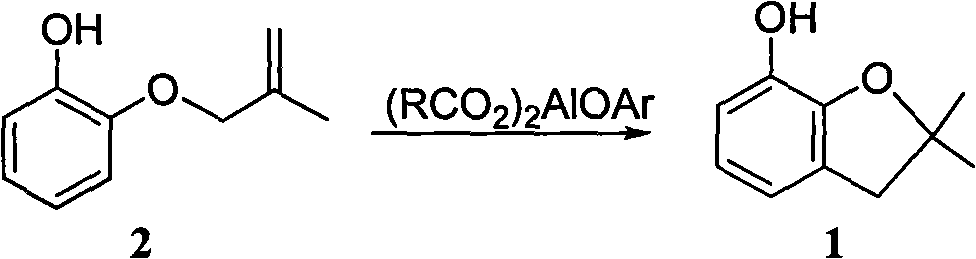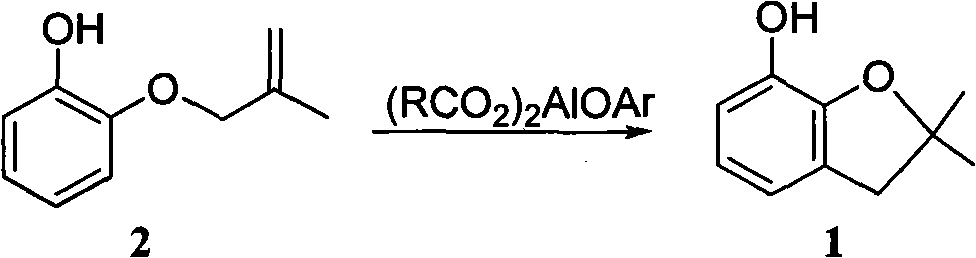Patents
Literature
75 results about "Ethoxybenzene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Packaging 100, 500 mL in glass bottle Application Ethoxybenzene (Phenetole) was used as an analyte in assaying the performance of the porous graphitic carbon (PGC) particles.
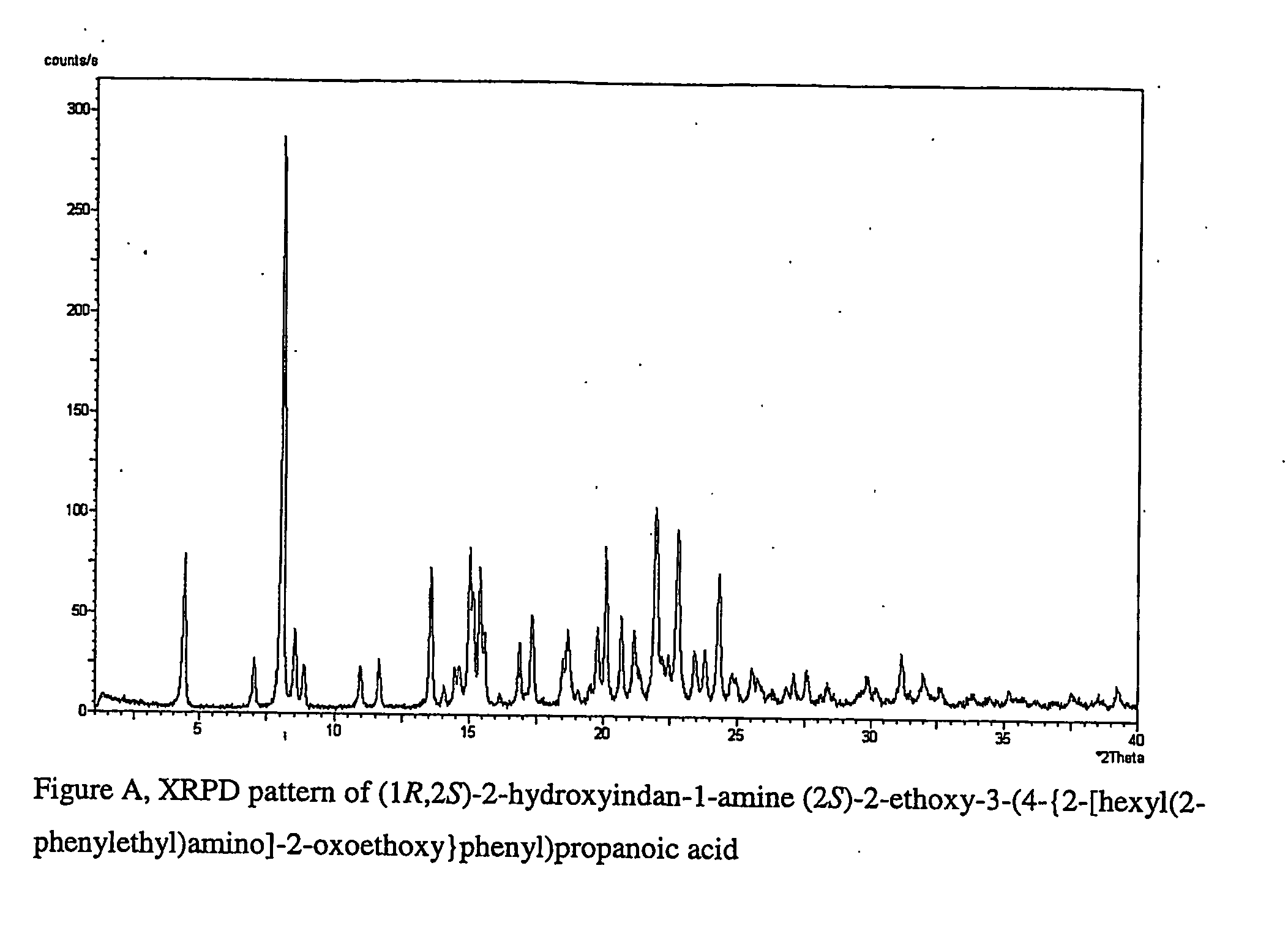
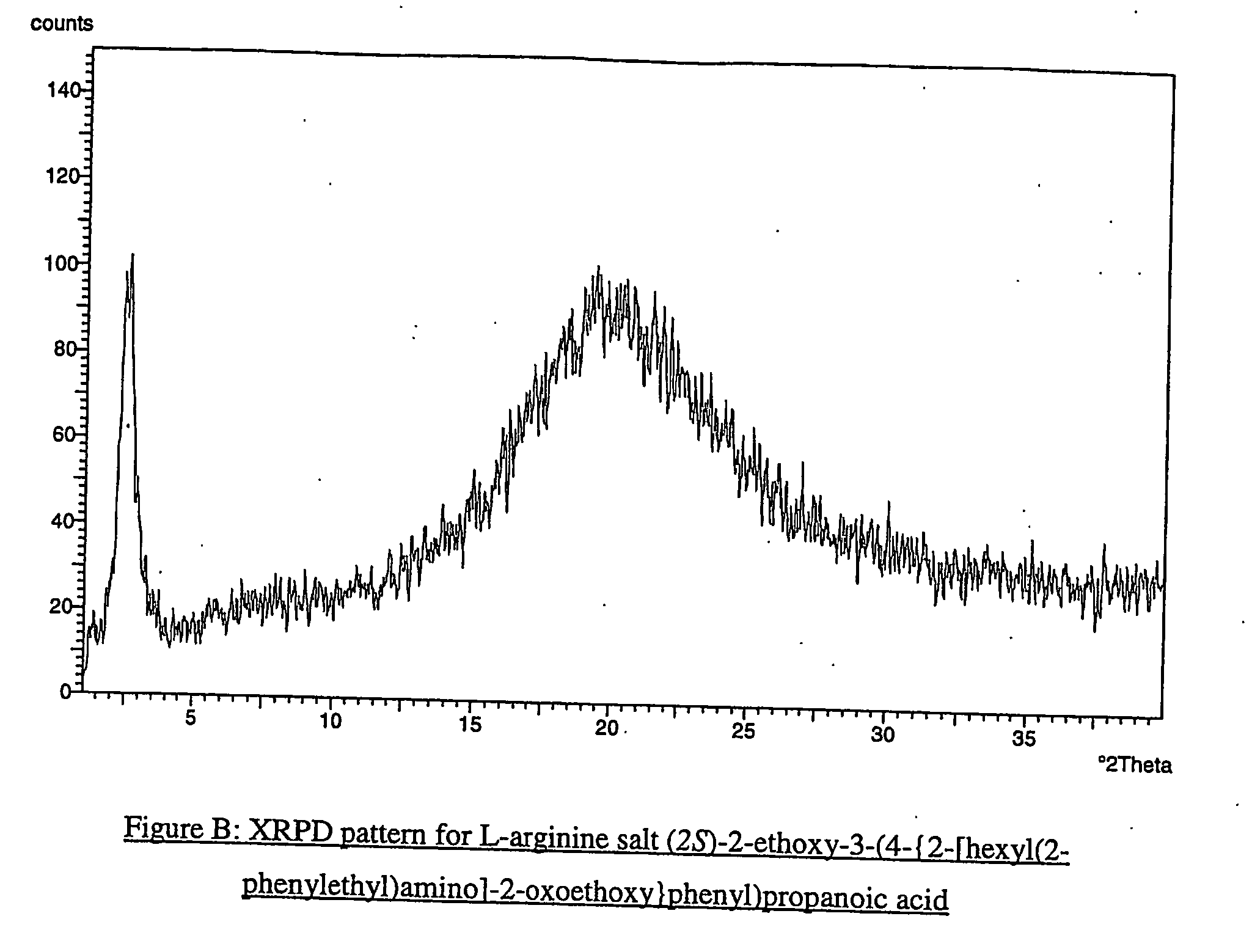
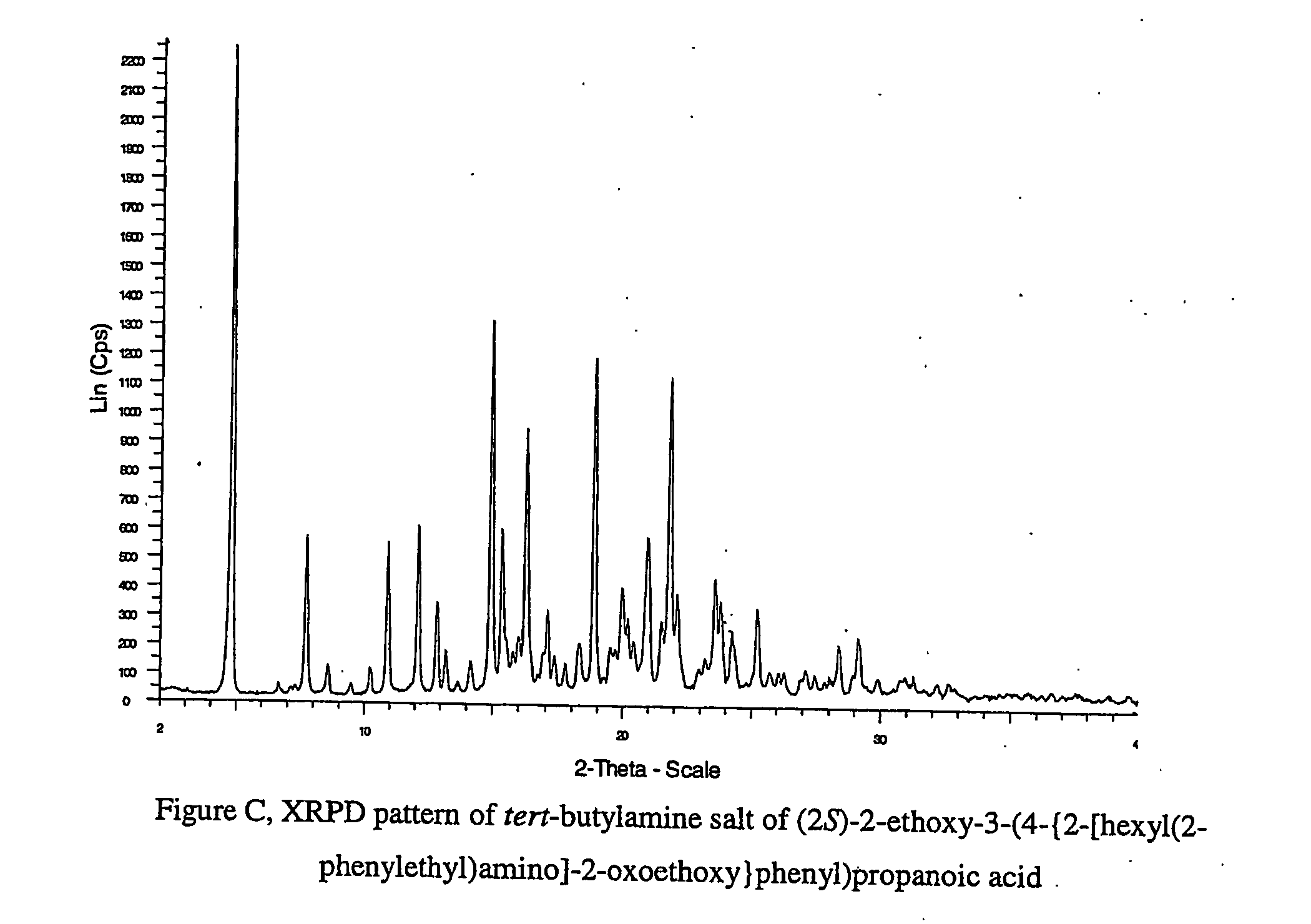
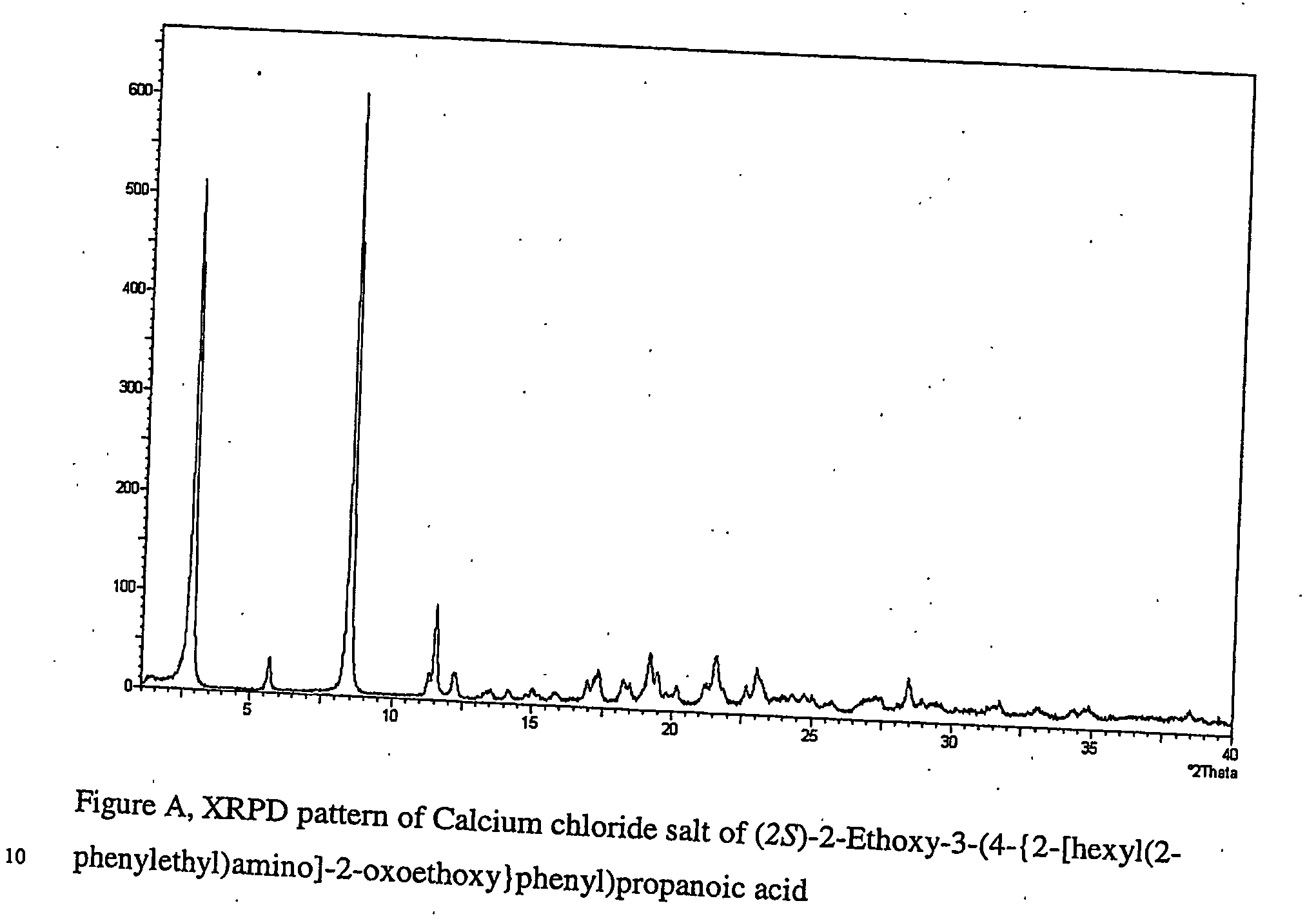
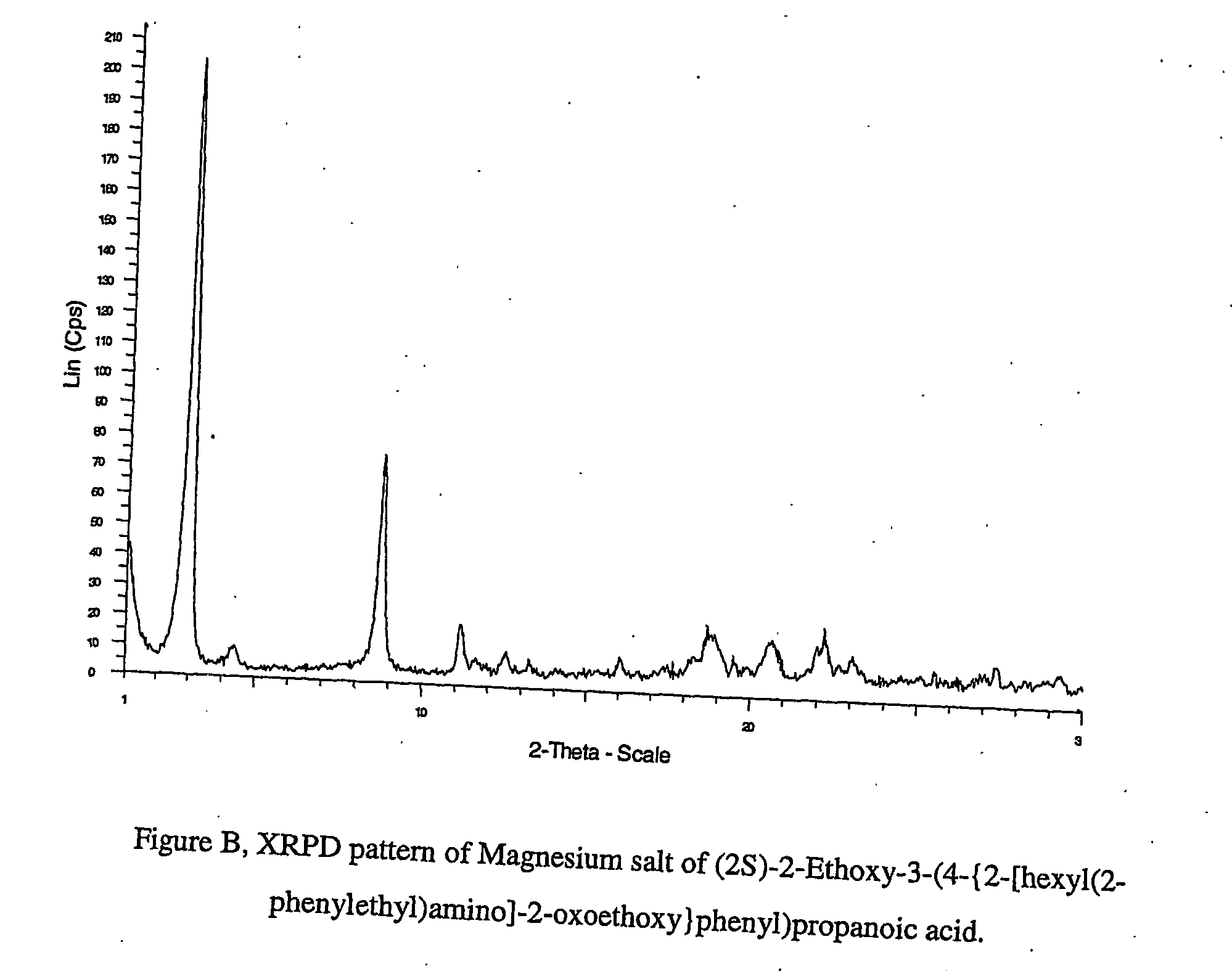
Pharmaceutically useful salts of carboxylic acid derivatives
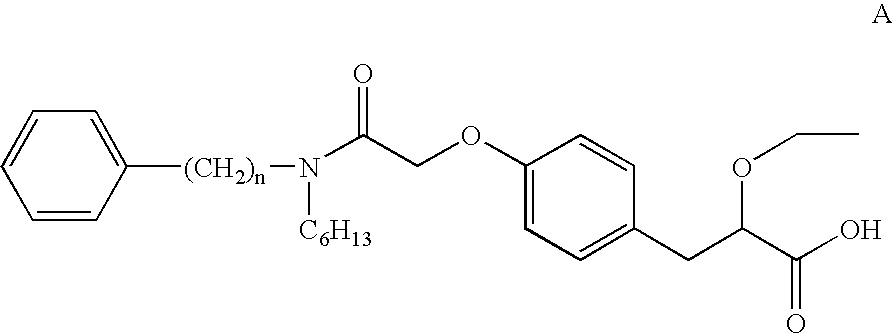
A compound selected from one or more of the following: a (1R,2S)-2-hydroxyindan-1-amine salt of (2S)-2-ethoxy-3-(4-{2-[hexyl(2-phenylethyl)amino]-2-oxoethoxy}phenyl)propanoic acid; an L-arginine salt of (2S)-2-ethoxy-3-(4-{2-[hexyl(2-phenylethyl)amino-2-oxoethoxyphenyl)propanoic acid; a tert-butylamine salt of (2S)-2-ethoxy-3-(4-{2-[hexyl(2-phenylethyl)amino-2-oxoethoxyphenyl)propanoic acid; a choline salt of (2S)-2-ethoxy-3-(4-{2-[hexyl(2-phenylethyl)amino]-2-oxoethoxy}phenyl)propanoic acid; an adamantylamine salt of (2S)-2-ethoxy-3-(4-{2-[hexyl(2-phenylethyl)amino]-2-oxoethoxy}phenyl)propanoic acid; a N-benzyl-2-phenylethanaminium salt of (2S)-2-ethoxy-3-(4-{2-[hexyl(2-phenylethyl)amino]-2-oxoethoxy}phenyl)propanoic acid; a N-benzyl-2-(benzylamino) ethanaminium salt of (2S)-2-ethoxy-3-(4-{2-[hexyl(2-phenylethyl)amino]-2-oxoethoxy}phenyl)propanoic acid; or a tris(hydroxymethyl)methylamine salt of (2S)-2-ethoxy-3-(4-{2-[hexyl(2-phenylethyl)amino]-2-oxoethoxy}phenyl)propanoic acid.
Owner:ASTRAZENECA AB
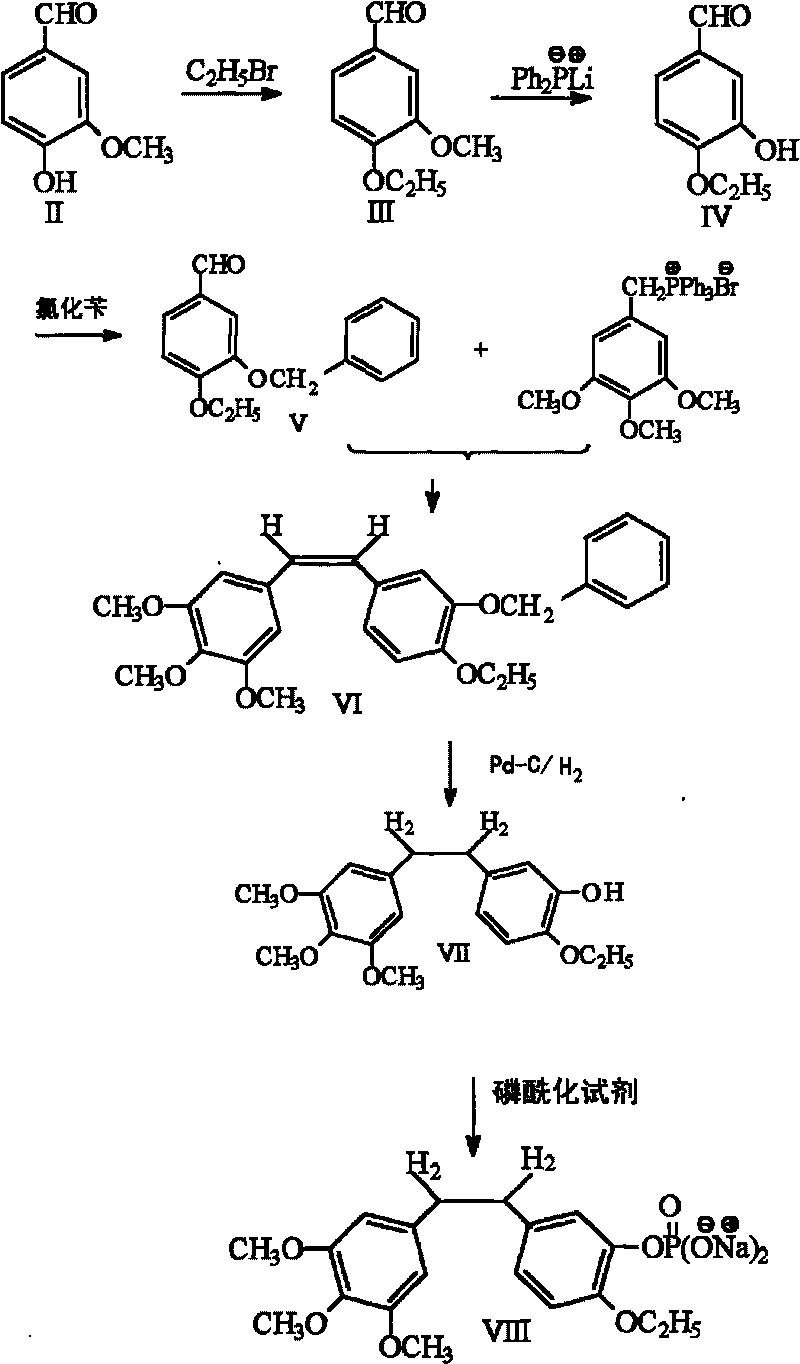
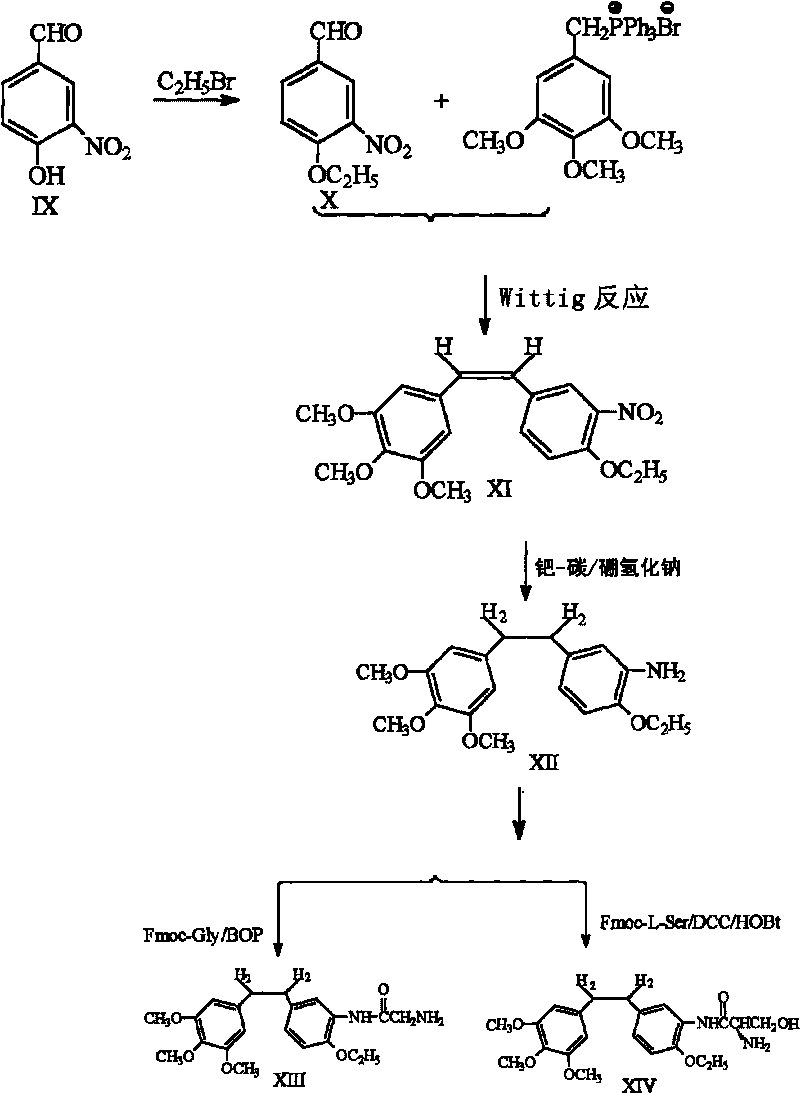
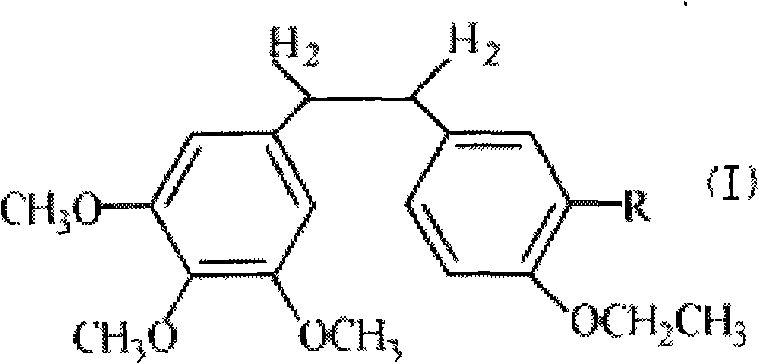
Ethoxy diphenyl ethane derivative and preparation method and application thereof
InactiveCN101723813AImprove stabilityReduce lossGroup 5/15 element organic compoundsEther/acetal active ingredientsAmino acid side chainLymphatic Spread
The invention discloses an ethoxy diphenylethane derivative and a preparation method and application thereof. The 4, position of a B aromatic ring of phenylethane is chemically modified by an ethoxyl group, and simultaneously, a hydroxyl group at the 3, position of the B aromatic ring of the phenylethane is modified into water-soluble pro-drugs such as phosphate and the like, and likewise, an amino acid side chain is introduced into an amino group at the 3, position to form an amino acid amide water-soluble pre-drug having a structure as shown by a structural formula (I). An ethoxy phenylethane derivative and a pre-drug thereof have strong capacity of inhibiting tubulin aggregation, have obvious targeted destructive functions to tumor vessels, selectively cause the tumor vessels to have dysfunctions and structural damages, induce the apoptosis of vascular endothelial cells so that tumor cells lose the support of nutrition and oxygen gas, and give play to the functions of killing and wounding the tumor cells or inhibiting the tumor metastasis.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD +1
Citric acid alidenafil crystal form B and preparation method and usage thereof
The invention relates to 1-[3-(6, 7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo [4, 3-d] pyridine-5-thyl)-4-ethoxylbenzene sulfonyl]-cis-3, 5-lupetazin citrate or citric acid alidenafil crystal form Band preparation method thereof and also relates to drug composistion containing citric acid alidenafil crystal form B and application thereof in preparation of drug for treating erection disturbance(ED). Citric acid alidenafil raw material is dissolved in distilled water in certain proportion, and heat preservation is carried out for 30-48 hours while stirring is carried out at certain temperature, thus obtaining the product. Test by an X-ray powder diffractometer, thermogravimetric analysis and infrared spectrometer proves that an unprecedented citric acid alidenafil crystal form B is obtained, the crystal form B can form a composition with one or multiple pharmaceutically acceptable carrier, excipient or diluent and can be effectively applied to treatment on andropathy.
Owner:刘桂坤
Citric acid aildenafil crystal form A and preparation method and usage thereof
The invention relates to 1-[3-(6, 7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazol parallel [4, 3-d] pyridine-5-thyl)-4-ethoxybenzene sulfonyl]-cis-3, 5-lupetazin citrate or citric acid alidenafil crystalform A and preparation method thereof and also relates to drug combination containing citric acid alidenafil crystal form A and application thereof in preparation of drug for treating erection disturbance. Citric acid alidenafil raw material is dissolved with distilled water in certain proportion, stirring is carried out for 60-80 minutes while temperature is maintained to be 38-42 DEG C, and stirring is carried out for 10-15 hours while temperature is maintained to be 20-26 DEG C, thus obtaining the product. Test by an X-ray powder diffractometer, thermogravimetric analysis and infrared spectrometer proves that an unprecedented citric acid alidenafil crystal form A is obtained, the crystal form A can form a combination with one or multiple pharmaceutically acceptable carrier, excipient or diluent and can be effectively applied to treatment on andropathy.
Owner:刘桂坤
Cleaning production method of 2-(Ethoxyethoxy) ethyl acrylate (EOEOEA) or phenoxyethyl acrylate (PHEA)
ActiveCN102633634ANo processing costsGreen and Clean Production MethodsOrganic compound preparationCarboxylic acid esters preparationSodium BentonitePhenyl Ethers
The invention belongs to the field of photocuring materials and relates to a cleaning production method of 2-(Ethoxyethoxy) ethyl acrylate (EOEOEA) or phenoxyethyl acrylate (PHEA). The method comprises the following steps: (1) carrying out esterification, backflow and dehydration on diethylene Glycol monoethylether or ethylene glycol phenyl ether, acrylic acid, a catalyst, a solvent, a polymerization inhibitor and an antioxidant; (2) adding caustic soda flakes and little water for neutralization; (3) adding a polymagnesium silicate adsorbent for adsorbing and neutralizing the generated salt; (4) reducing pressure for dehydration and removing the solvent; (5) carrying out filter pressing so as to filter polymagnesium silicate and the salt adsorbed by polymagnesium silicate; (6) adding alkaline Ca-based bentonite and calcium oxide for decoloration, micro water removal and trace acid removal; (7) carrying out filter pressing; and (8) detecting product indexes. The cleaning production method disclosed by the invention has high yield and social and environmental benefits, thus thoroughly solving the organic waste water pollution problem during the production process of EOEOEA or PHEA.
Owner:JIANGSU LITIAN TECH
Citric acid alidenafil crystal form D and preparation method and usage thereof
The invention relates to 1-[3-(6, 7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazole parallel [4, 3-d] pyridine-5-thyl)-4-ethoxylbenzene sulfonyl]-cis-3, 5-lupetazin citrate or citric acid alidenafil crystal form D and preparation method thereof and also relates to drug combination containing citric acid alidenafil crystal form D and application thereof in preparation of drug for treating erection disturbance. Citric acid alidenafil raw material is dissolved in distilled water / acetone mixed solution in certain proportion, and heat preservation is carried out for 50-72 hours while stirring is carried out at certain temperature, thus obtaining the product. Test by an X-ray powder diffractometer, thermogravimetric analysis and infrared spectrometer proves that an unprecedented citric acid alidenafil crystal form D is obtained, the crystal form D can form a combination with one or multiple pharmaceutically acceptable carrier, excipient or diluent and can be effectively applied to treatment on andropathy.
Owner:刘桂坤
Preparation of r-5-(2-(2-ethoxyphenoxyethylamino)propyl)-2-methoxybenzenesulphonamide hydrochloride of high chemical purity
Owner:LEK PHARMA D D
Aildenafil citrate crystal form O, preparation method and application thereof
InactiveCN101830903ASuitable for long term storageCrystal stableOrganic active ingredientsOrganic chemistry methodsDiseaseMethyl group
The invention relates to 1-[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazol[4,3-d] pyridine-5-group)-4-ethoxybenzene sulfonyl]-cis-3,5-lupetazin citrate or aildenafil citrate crystal form O and a preparation method thereof. The invention also relates to a medicine compound containing aildenafil citrate crystal form O and the application thereof in preparing the medicine for treating erectile dysfunction (ED). The aildenafil citrate crystal form O is formed by the steps of: solving citrate in the distilled water and tetrahydrofuran, stirring, rising temperature, filtering, stirring filtrate, decreasing temperature, crystallizing and filtering. The aildenafil citrate crystal form O is prepared into the medicine together with the medical excipients and is applied in treating the erectile dysfunction disease.
Owner:HONG KONG GOLDEN WISDOM MEDICAL PRODN LTD
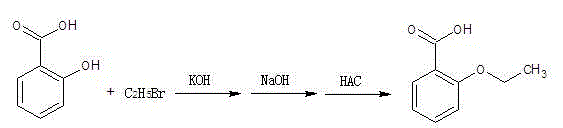
Preparation method of o-ethoxybenzoic acid
ActiveCN103553908ALow costSimple processPreparation from carboxylic acid saltsOrganic compound preparationAcetic acidFiltration
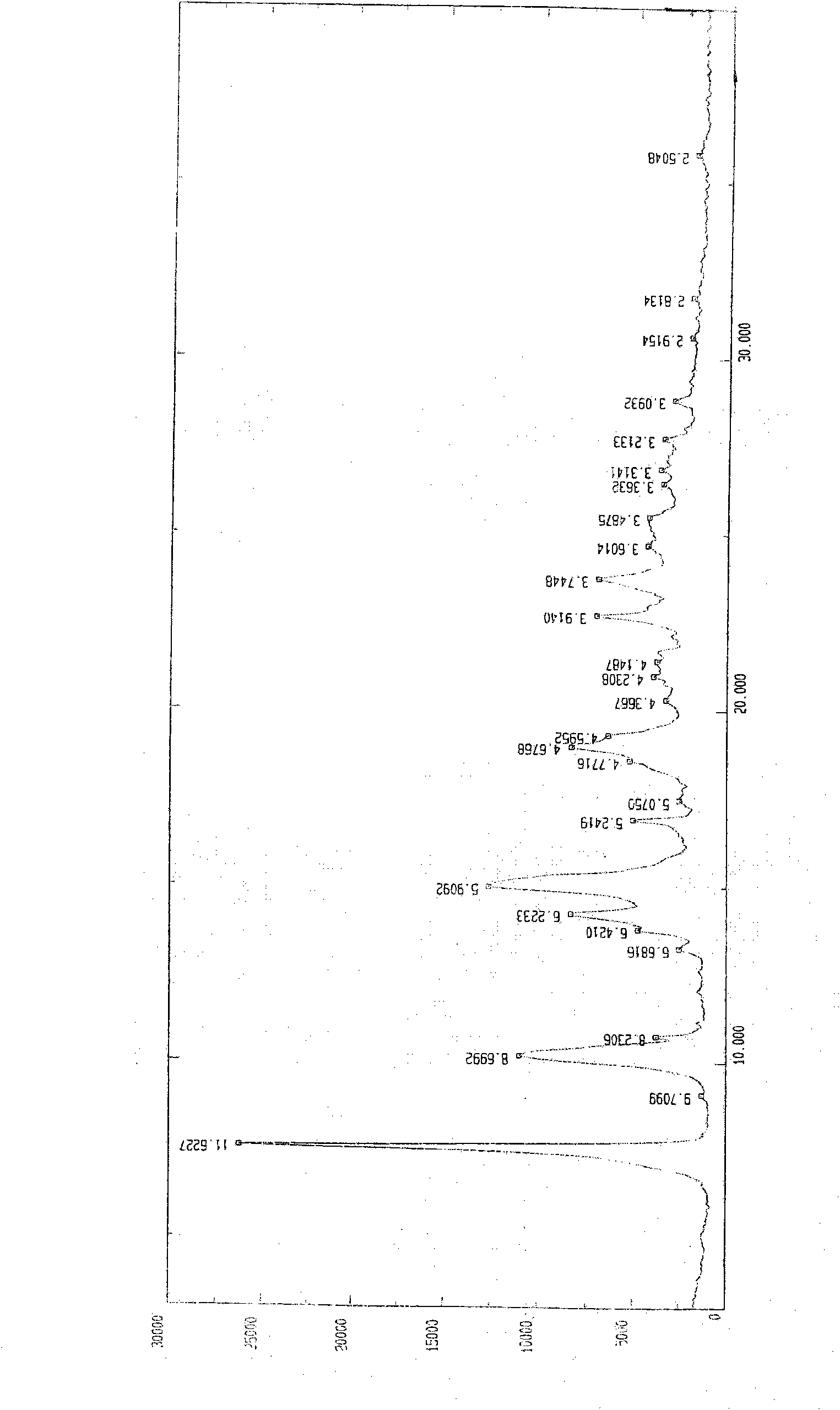
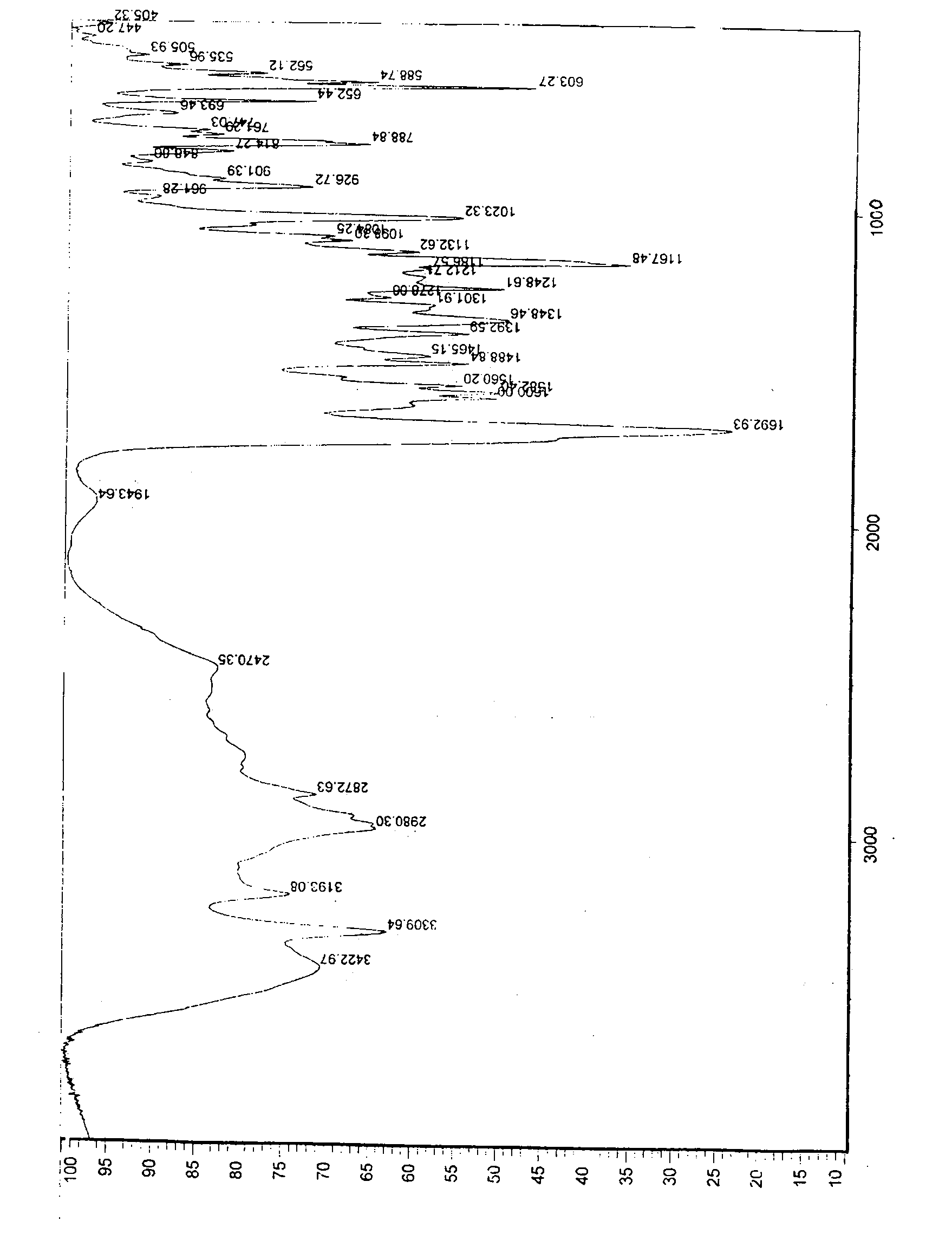
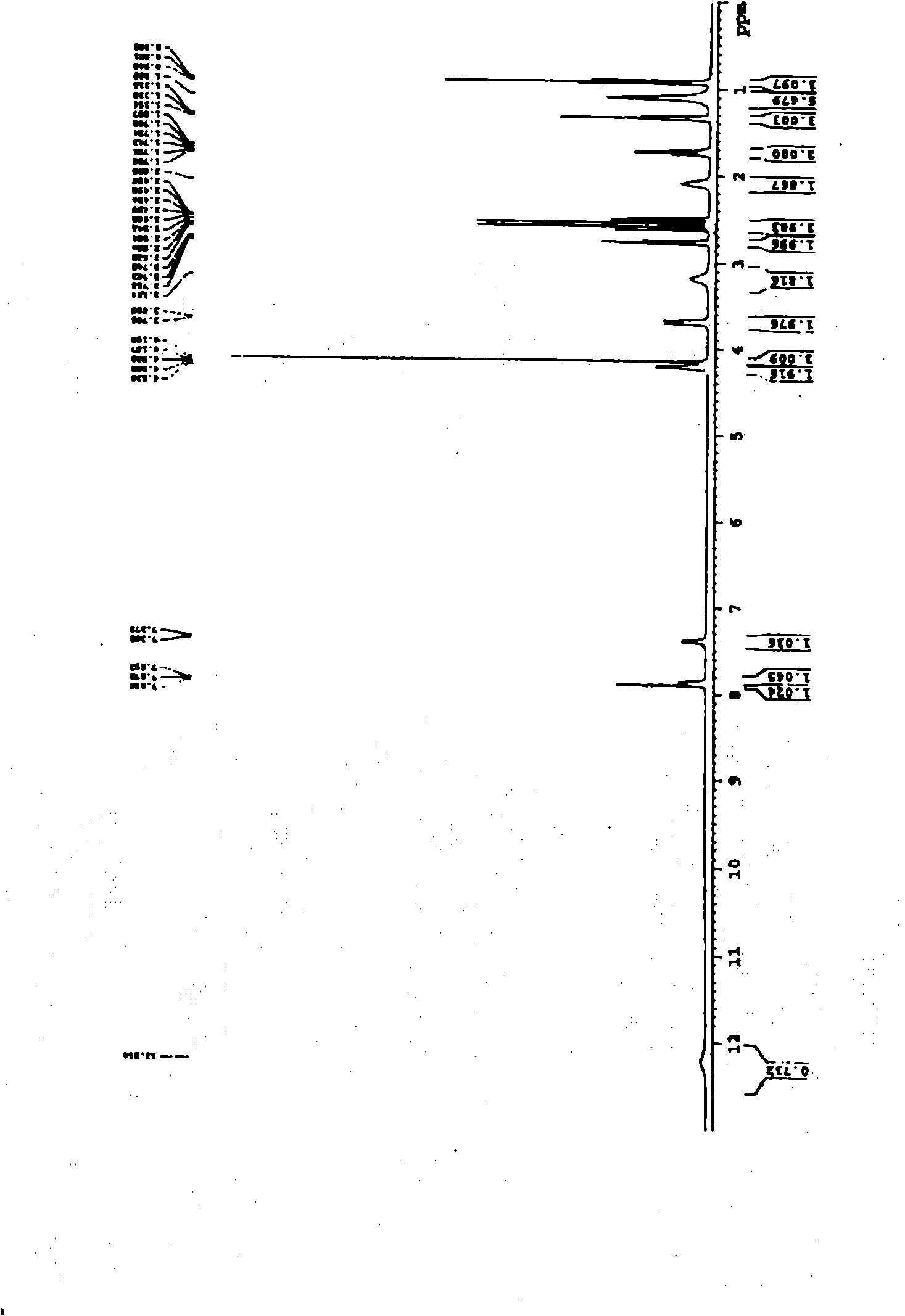
The invention relates to a preparation method of o-ethoxybenzoic acid, which comprises the following steps: adding salicylic acid, acetone and potassium hydroxide into a reaction bulb, dropwisely adding bromoethane while stirring at room temperature, heating under reflux for 9-15 hours, recovering the acetone under reduced pressure, adding water and liquid alkali, heating under reflux for 2-4 hours, cooling to room temperature, regulating the pH value to 3-4 with glacial acetic acid, cooling to 1-15 DEG C, separating by vacuum filtration, washing with water, and carrying out vacuum drying to obtain the o-ethoxybenzoic acid, wherein the salicylic acid:potassium hydroxide:bromoethane:liquid alkali mol ratio is 1:(2.0-2.5):(2.1-2.5):1.4. The method has the advantages of simple technique, low raw material cost, high yield and good quality, and is convenient to operate and convenient for industrial production.
Owner:苏州诚和医药化学有限公司
Method for synthesizing Vardenafil hydrochloric acid
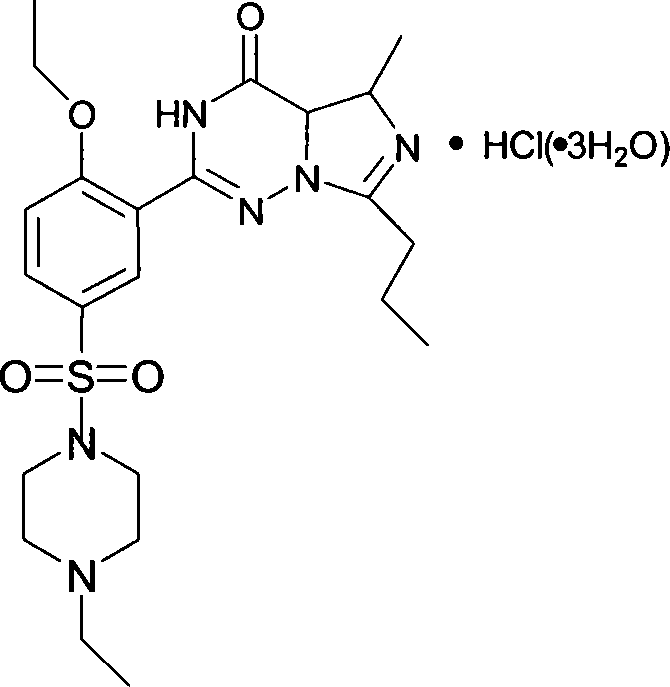
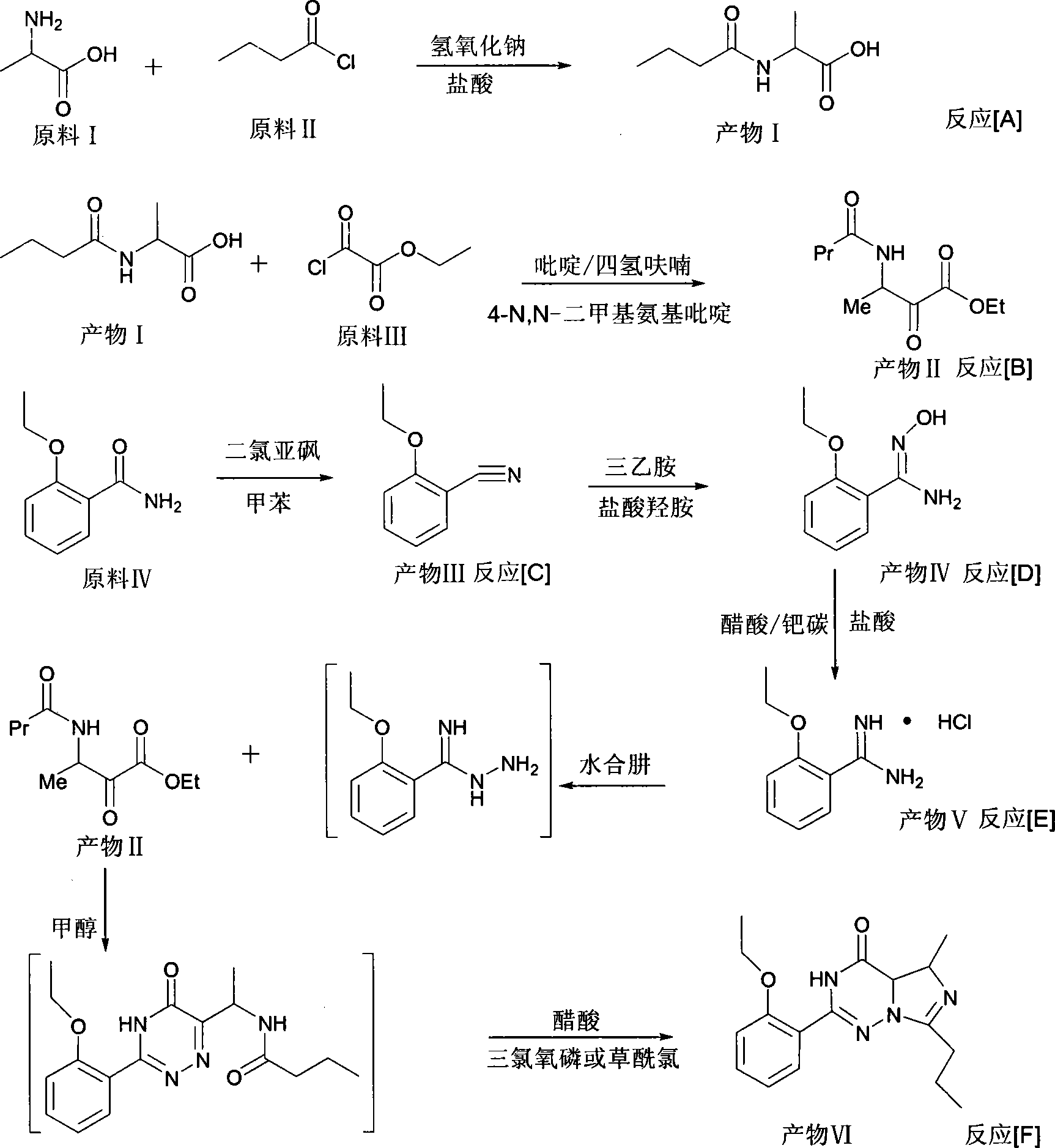
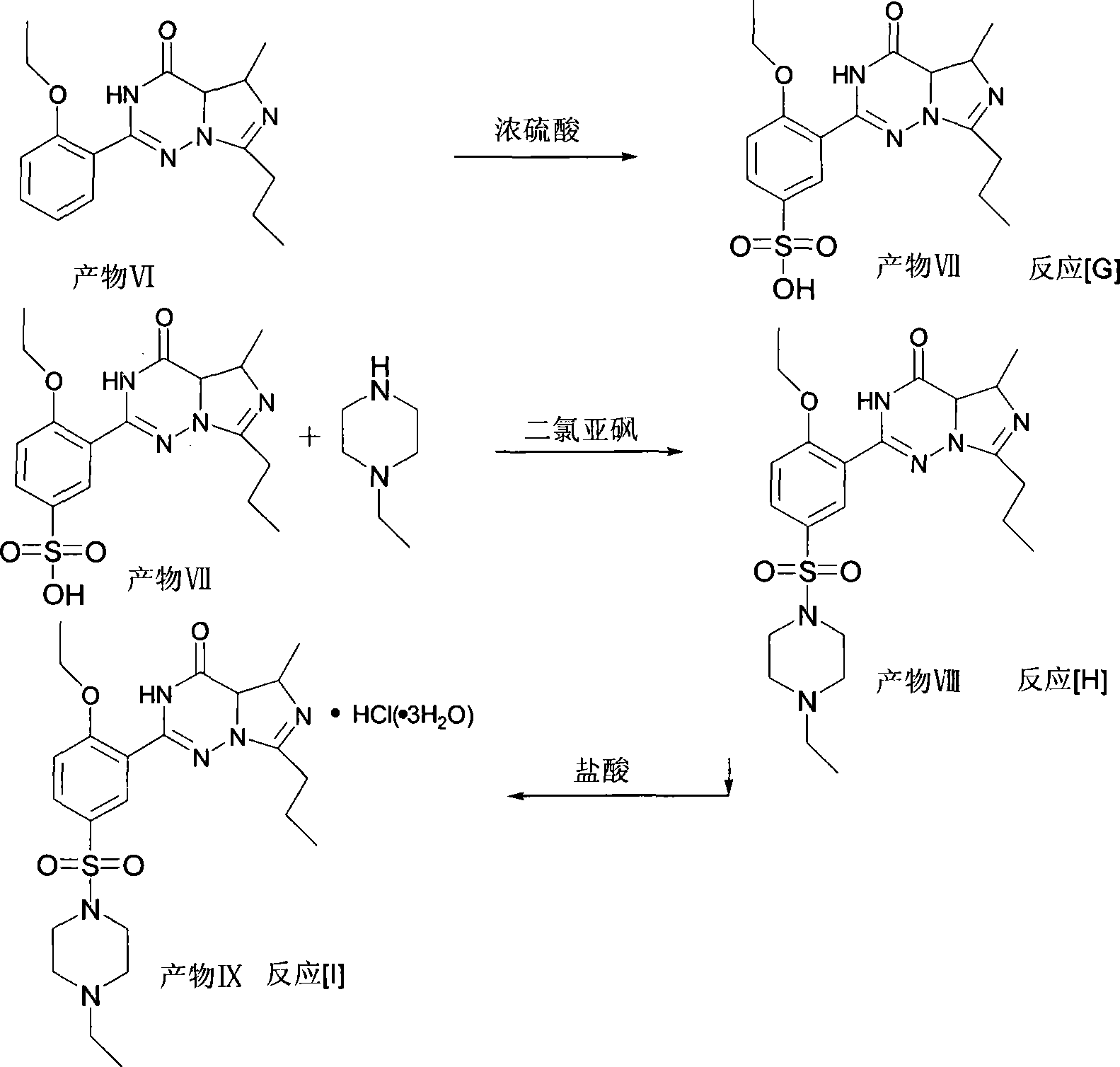
This invention relates to a method for synthesizing vardenafil hydrochloride. The method comprises: (1) performing acylation and nucleophilic addition on D,L-alanine raw material, condensing with 2-ethoxybenzamidine hydrochloride twice, and performing ring-closing reaction to obtain 2-(2-ethoxyphenyl)-5-methyl-7-propyl-3H-imidazo[5,1-f][1,2,4]-trizin-4-one; (2) performing sulfonation reaction to obtain 4-ethoxy-3-(3,4-dihydroxy-5-methyl-4-oxo-7-propyl imidazo[1,5-f][1,2,4]trizin-4-one)benzenesulfonic acid; (3) reacting with N-ethyl pyrazine to obtain vardenafil, and reacting with HCl to obtain vardenafil hydrochloride. The method has such advantages as easy operation, high yield, and simple process and low reaction condition requirement, thus is suitable for industrialization.
Owner:BEIJING UNIV OF CHEM TECH
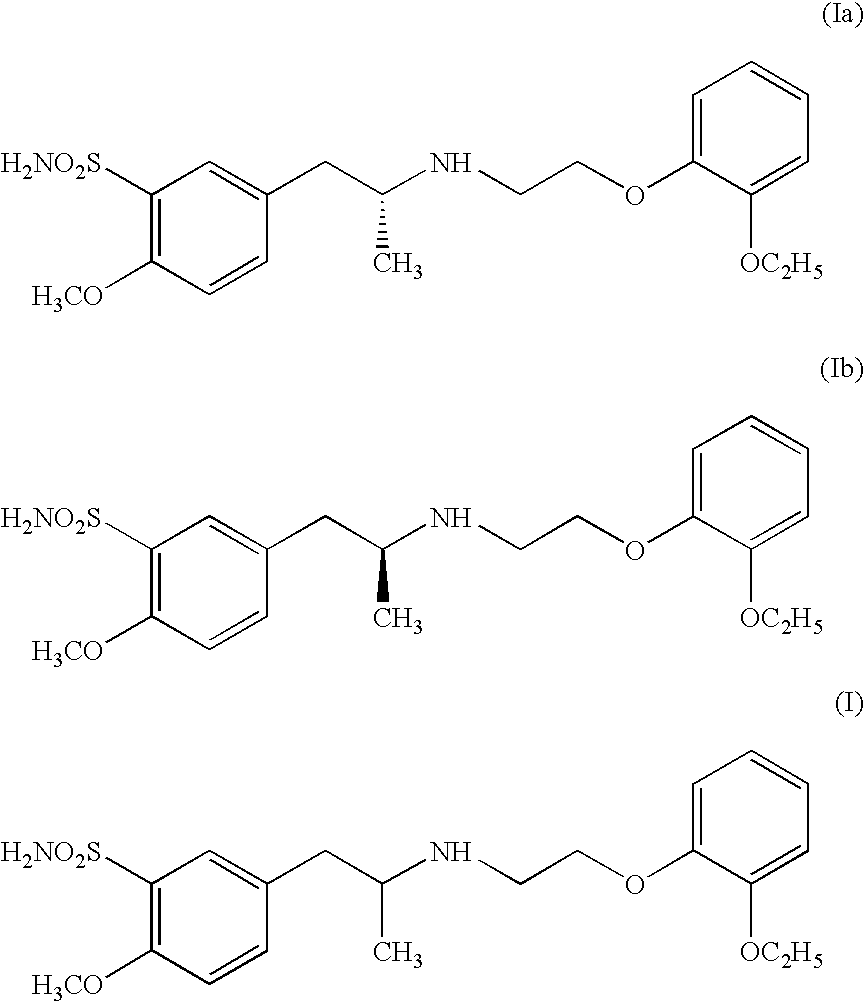
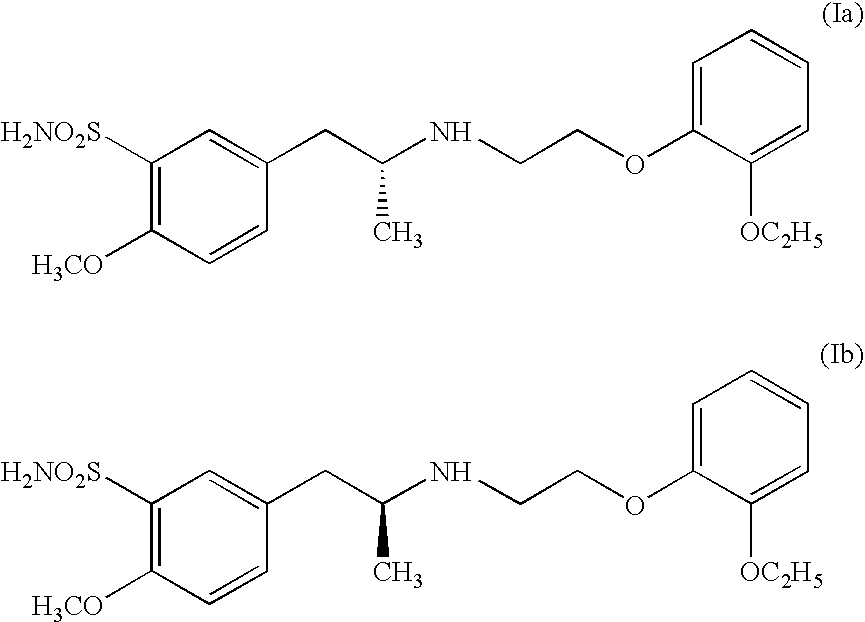
Process for preparing R- and S-isomers of (R)-5-(2-( (2-(2-ethoxyphenoxy) ethyl) amino) propyl) -2-methoxybenzenesulfonamide
InactiveUS20050079589A1Organic compound preparationOrganic chemistry methodsOrganic solventEnantiomer
A process for preparing optically pure enantiomers of R-(−)tamsulosin of formula Ia and S-(+)tamsulosin of formula Ib by resolving racemic tamsulosin of formula I by means of (1R)-(−)-camphor-10-sulfonic acid and (1S)-(+)-camphor-10-sulfonic acid, resp., in an environment of organic solvents, water or mixtures thereof.
Owner:FARMAK
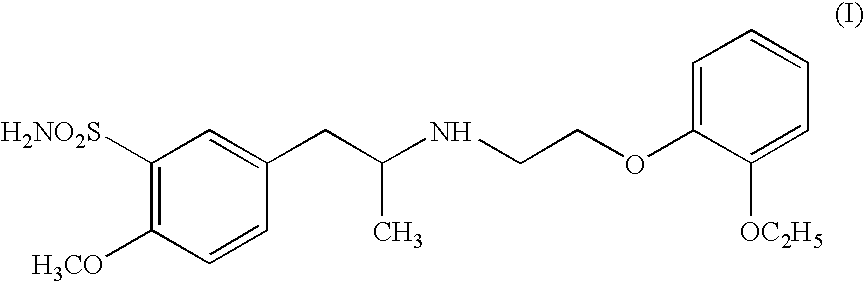
Pharmaceutically useful salts of carboxylic acid derivates
A calcium or a magnesium salt of (2S)-2-ethoxy-3-(4-{2[hexyl(2-phenylethyl)amino]-2-oxoethoxy}phenyl)propanoic acid.
Owner:ASTRAZENECA AB
Aildenafil citrate crystal form C and preparation method and application thereof
The invention relates to 1-[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo [4,3-d] pyrimidine-5-yl)-4-ethoxybenzenesulfonyl]-cis-3,5-dimethyl-piperazine citrate or a aildenafil citrate crystal form C and a preparation method thereof. The invention also relates to a medicinal composition containing the aildenafil citrate crystal form C and application thereof in preparing medicaments for treating male erection disturbance. An unprecedented aildenafil citrate crystal form C is proved to be obtained through the steps of dissolving an aildenafil citrate raw material in a mixed solution of distilled water and acetone at a certain proportion, keeping the temperature for 8 to 10 hours at a certain temperature while stirring, and testing the prepared product by an X-ray powder diffractometer, a thermogravimetric analyzer and an infrared spectrometer; and the crystal form C can form a composition together with one or more pharmaceutically acceptable carriers, an excipient or a diluent, and can be effectively applied to the treatment of andropathy.
Owner:刘桂坤
Crystal form V of Aildenafil citrate and preparation method and application thereof
InactiveCN101698668AGood chemical stabilitySuitable for long term storageOrganic active ingredientsOrganic chemistryAildenafilMethyl group
The invention relates to a crystal form V of 1-[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H- pyrazolo[4,3-d] 5-pyrimidinyl)-4-ethyoxylbenzenesulfonyl]-cis-3,5-dimethylpiperazine citrate or Aildenafil citrate, and a preparation method thereof. The preparation method comprises the following steps: dissolving raw materials of the Aildenafil citrate in distilled water, stirring, and raising temperature to a reflux temperature; and adding acetone solution, and stirring and raising temperature continuously. The prepared product meets product requirements after being tested by an X-ray powder diffractometer and an infrared spectrometer. The invention also relates to a medicinal composition comprising the crystal form V of the Aildenafil citrate, and application of the crystal form V of the Aildenafil citrate in preparing medicaments for treating erection disturbance (ED).
Owner:刘桂坤
Preparation method and application of fluorine-containing microspheres without stabilizers on surfaces
The invention discloses a preparation method and an application of fluorine-containing microspheres without stabilizers on the surfaces. Anhydrous THF (tetrahydrofuran) is added to a single-neck flask, mPEG (methoxy polyethylene glycol), an RAFT reagent CEPDB (4-(2-carboxyethylcarbonyl)oxy-ethoxy phenyl dithiocarbamate isobutyronitrile ester) and DMAP (4-dimethylaminopyridine) are dissolved in anhydrous THF, the single-neck flask is placed in an ice-water bath, and the mixture is stirred and cooled to 0 DEG C; DCC (dicyclohexylcarbodiimide) is dissolved in anhydrous THF, and a DCC-THF solution is obtained; the DCC-THF solution is all dropwise added to the single-neck flask within 30-50 min, and the single-neck flask is continuously placed in the ice-water bath for a reaction for 1 h after the DCC-THF solution is dropwise added. The method is rapid, environment-friendly and energy-saving, and fluorine-containing polymer microspheres without stabilizers on the surfaces are rapidly prepared with an optical RAFT dispersion polymerization method and a thermal aftertreatment method with an ethanol / water mixed solvent as a reaction medium. The preparation method is simple and the reaction speed is high.
Owner:FOSHAN UNIVERSITY
Cell culture apparatus and a fabricating method of the same
ActiveUS20090004731A1Good biocompatibilityEasy to controlBioreactor/fermenter combinationsBiological substance pretreatmentsGlycidyl methacrylateTriethyleneglycol dimethacrylate
A cell culture apparatus and a method for fabricating the cell culture apparatus are disclosed, the method comprises forming at least one fillister on a biomaterial composite layer by photolithography, wherein the biomaterial composite layer contains two gel materials. One is a bio-compatible hydrogel composition having various weight ratio of: 2-hydroxyethylmathacrylate (HEMA), bisphenol A and glycidyl methacrylate (bis-GMA), triethylene glycol dimethacrylate (TEGDMA), r-methacryloxypropyl trimethoxysilane (MAPTMS), α,α-diethoxyacetophenone (DEAP), and the other one is a photo-sensitive silica gel composition.
Owner:IND TECH RES INST
Composite ferrite nanoparticles for synergistically enhancing liver specificity as well as preparation method and application thereof
PendingCN111821473AGuaranteed accuracyEnsure safetyMaterial nanotechnologyNanomagnetismFerrite nanoparticlesPhenyl group
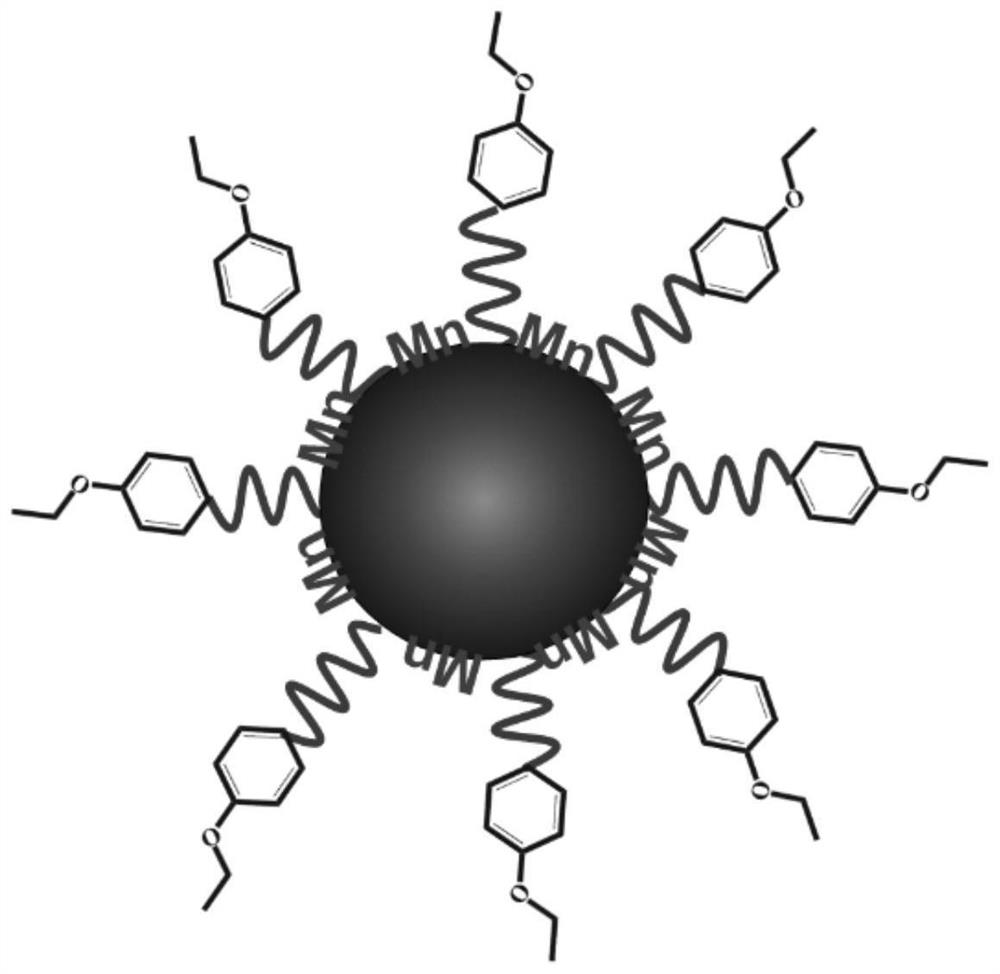
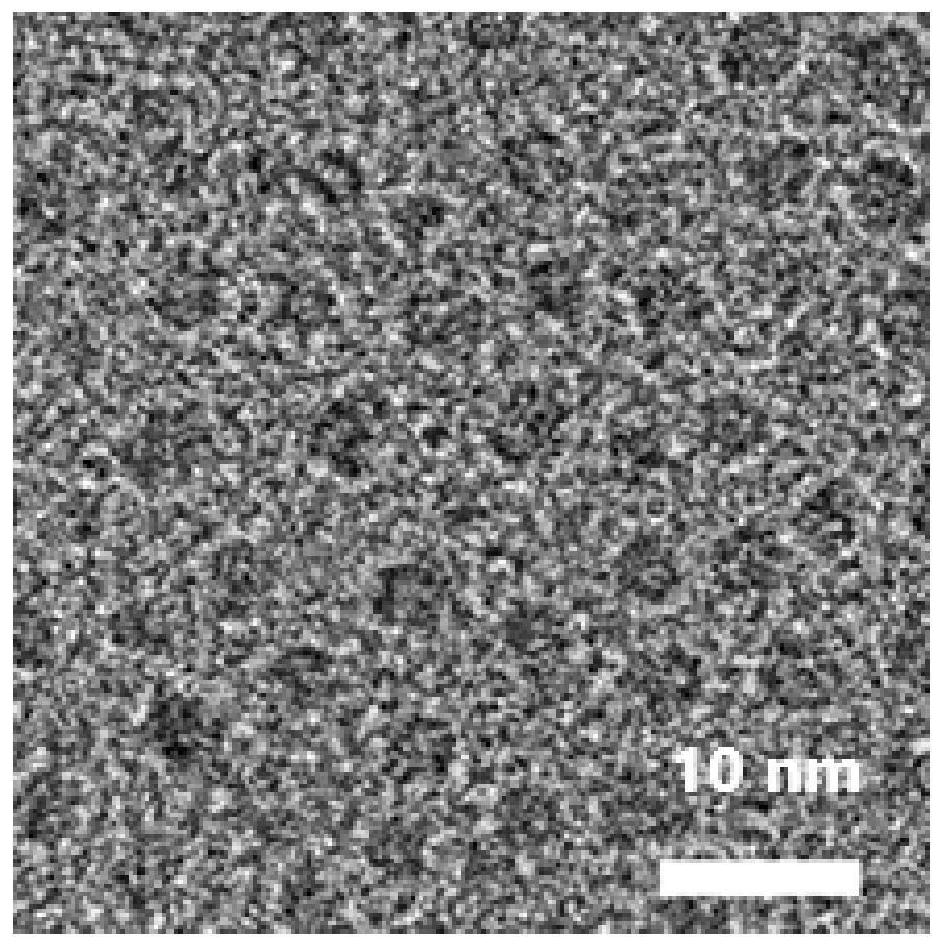
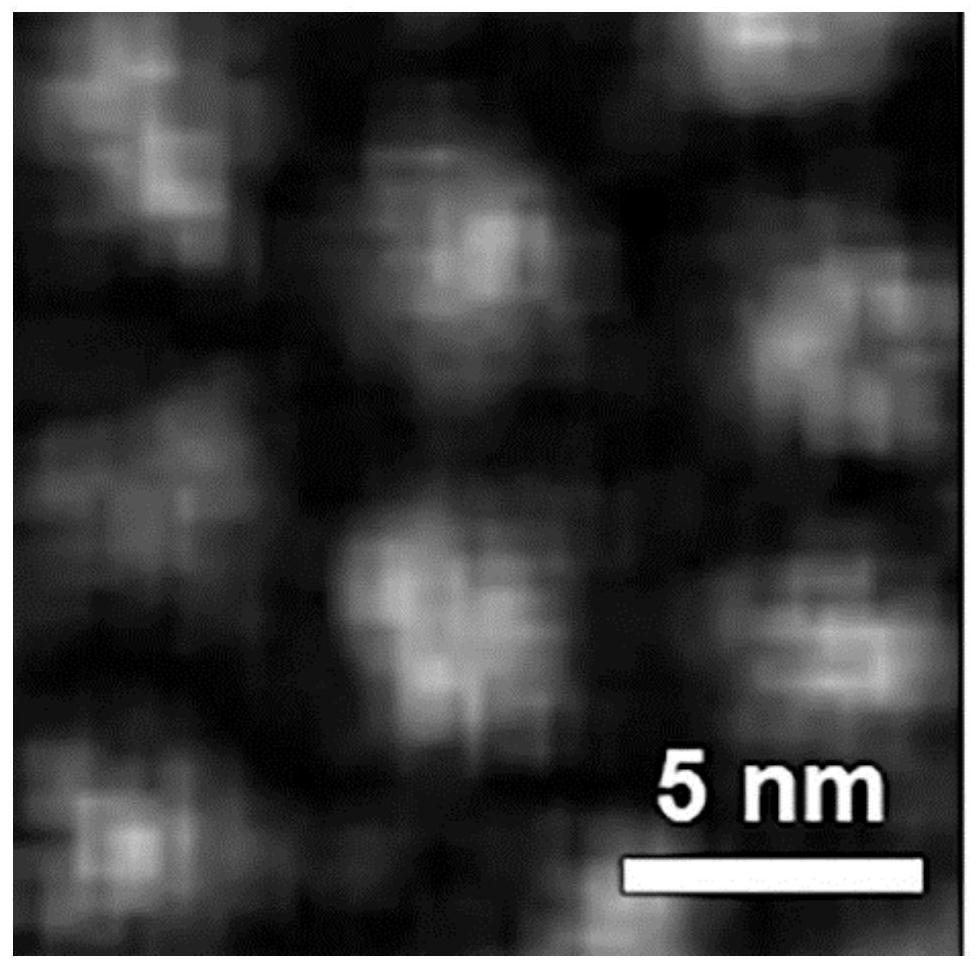
The invention discloses composite ferrite nanoparticles for synergistically enhancing liver specificity. The composite ferrite nanoparticles contain manganese ions and ethoxyphenyl groups at the sametime, the mole percentage of the ethoxyphenyl groups and the manganese ions is 25-60%, the molar percentage of the manganese ions and iron ions in the ferrite nanoparticles is 40-80%, the particle size range of the composite ferrite nanoparticles with the manganese ions and the ethoxyphenyl groups on the surfaces is 0.2-5 nm, and the preferential particle size range is 2-4 nm. The invention also discloses a preparation method of the composite ferrite nanoparticles and an application of the composite ferrite nanoparticles in preparation of a T1 developer in magnetic resonance imaging. The composite ferrite nanoparticles provided by the invention enhance the hepatocyte specificity due to the synergistic effect of the manganese ions and the ethoxyphenyl groups, so that high-specificity T1 enhanced imaging of the liver in MRI imaging is realized.
Owner:XIAN SUPERMAG BIO NANOTECH CO LTD
Preparation method of 3-ethoxy-4-hydroxymandelic acid used as intermediate for synthesizing ethyl vanillin
InactiveCN102190580AHigh yieldReduce generationOrganic compound preparationCarboxylic compound preparationGlyoxylic acid3-ethoxy-4-hydroxymandelic acid
The invention discloses a preparation method of 3-ethoxy-4-hydroxymandelic acid, which is an intermediate used in the synthesis of ethyl vanillin. The preparation method comprises the following steps: adding water into glyoxalic acid, well-mixing the mixture, and using a sodium hydroxide solution to regulate the pH value of the mixture to 5.0 to 5.5, such that a glyoxalic acid solution is obtained; adding water into 2-ethoxyphenol, well-mixing the mixture, and using the sodium hydroxide solution to regulate the pH value of the mixture to 10 to 12 under a temperature of 25 to 30 DEG C, such that a 2-ethoxyphenol solution is obtained; simultaneously dropping the glyoxalic acid solution and the sodium hydroxide solution into the 2-ethoxyphenol solution under a temperature of 25 to 30 DEG C, than heating the mixture to a temperature of 50 to 60 DEG C, stirring for 1 to 3 hours with the temperature maintained, such that 3-ethoxy-4-hydroxymandelic acid is obtained. According to the invention, through an optimized arrangement of reaction temperatures, the reaction time is substantially reduced, and the production yield is substantially increased.
Owner:CHONGQING THRIVE CHEM
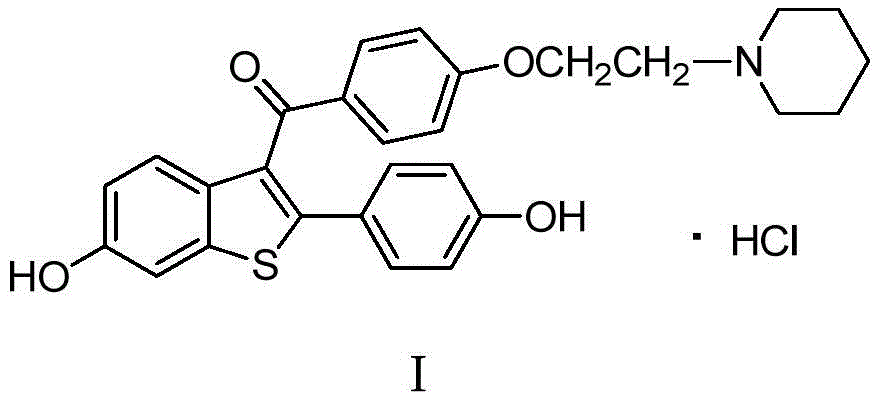
Preparing method for osteoporosis preventing medicine
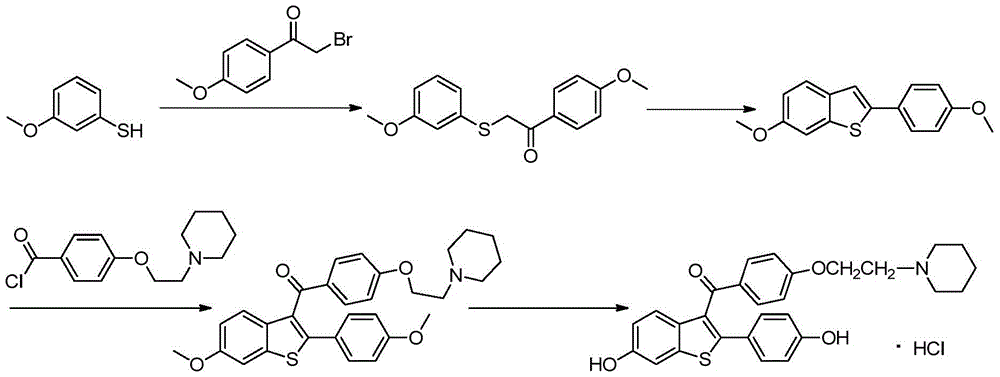
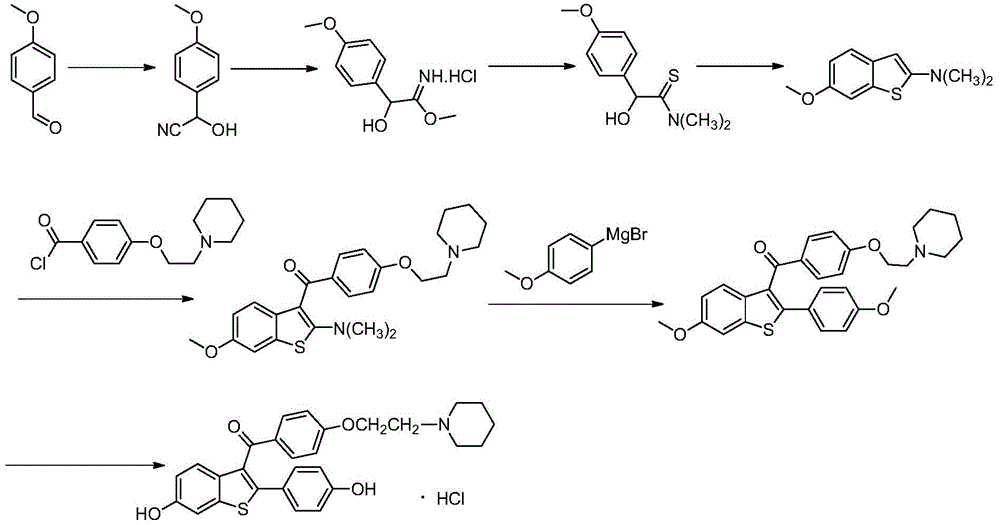
The invention relates to a preparing method for osteoporosis preventing medicine. Particularly, the invention relates to a preparing method for [6-hydroxy-2-(4-hydroxyphenyl) benzo-[b] thiophene-3-base] [4-[2-(1-piperidyl) ethyoxyl] phenyl] ketone hydrochloride. According to the preparing method, 2-halogenate-6-metoxybenzene thiophthene (II) as a beginning raw material, and a target product (I) can be prepared through coupling, fridel-crafts reaction, deprotection and salifying. The method has the advantages of being shorter in synthetic route, simple to operate, moderate in condition, high in efficiency and suitable for industrial production. (see the description).
Owner:SHANGHAI TIANCI BIOLOGICAL VALLEY BIOLOGICAL ENG
Preparation method for nanocapsule
InactiveCN105195069ASmall particle sizeCheap and easy to getPharmaceutical non-active ingredientsMicroballoon preparationMicro nanoOil phase
The invention provides a preparation method for a nanocapsule. The preparation method is characterized by comprising the following steps: (1) subjecting water, surfactant, cosurfactant and an oil phase to a stirring reaction at 10 to 80 DEG C for 2 min to 5 h so as to obtain a stable transparent microemulsion; (2) adding ethyl orthosilicate and ammonia water, carrying out a hydrolysis reaction for 0.1 to 12 h, then adding N-isopropyl acrylamide, polyacrylic acid and a photoinitiator diethoxyacetophenone and carrying out a reaction for 0.1 to 12 h so as to obtain micro-nano spheres; (3) subjecting the micro-nano spheres to ultraviolet irradiation so as to initiate polymerization of function groups; and (4) removing silica particles so as to obtain the nanocapsule. Compared with the prior art, the invention has the following advantages: since a reversed-phase microemulsion process is employed to prepare a template, the nanocapsule has a small particle size; and poly(N-isopropyl acrylamide) and polyacrylic acid are used as capsule wall materials, so double responsiveness to a pH value and temperature is obtained.
Owner:NINGBO UNIVERSITY OF TECHNOLOGY
O-ethoxyl phenol synthesizing process
The o-ethoxyl phenol synthesizing process with catechol and ethyl chloride as material includes the following steps: adding toluene and water into high pressure reactor, starting stirrer and water cooling the shaft of the high pressure reactor; adding catechol, sodium carbonate, alkyl amine bromide catalyst, solid sodium hydroxide, ethyl chloride in required amount successively; sealing, heating and maintaining at 100-139 deg.c and 0.1-1.1 MPa for 2-6 hr. The weight proportion among catechol, sodium hydroxide, sodium carbonate, ethyl chloride, alkyl amine bromide, water and toluene is 1 to 0.364-0.509 to 0.045-0.273 to 0.591-0.938 to 0.0091-0.0682 to 1.591-2.730 to 1.820-4.545. The present invention has yield of 86 %, and compared with other process, the present invention has lowered product cost, less sewage exhaust, low sewage COD, short technological process and less investment.
Owner:CNPC JILIN CHEM GROUP CORP
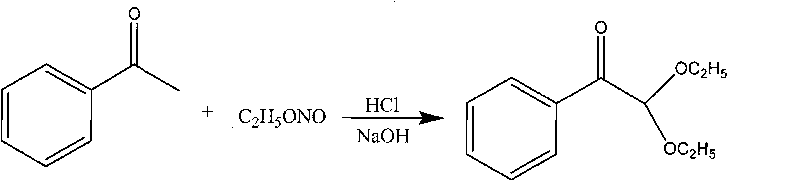
Synthetic method of 2,2-diethoxy acetophenone photoinitiator
InactiveCN101735031AAvoid decompositionEase of industrial productionOrganic compound preparationCarbonyl compound preparationAcetophenoneSodium nitrite
The invention relates to a synthetic method of a 2,2-diethoxy acetophenone photoinitiator, which comprises the following steps: a, synthesizing ethyl nitrite, namely mixing ethanol with 28 percent of aqueous solution of sodium nitrite in a reaction kettle with mechanical stirring and a gas ingress pipe, dripping 20 percent dilute sulphuric acid into the mixture at the temperature of between 20 and 30 DEG C to generate ethyl nitrite gas, and directing introducing the generate ethyl nitrite gas into the step b for reaction, wherein the molar ratio of the ethanol to the sodium nitrite to the dilute sulphuric acid is 1.00:1.09 to 1.68:1.10 to 1.18; and b, synthesizing 2,2-diethoxy acetophenone, namely introducing the ethyl nitrite gas generated by the step a into acetophenone and 36 percent hydrogen chloride ethanol solution, and neutralizing the mixture by using sodium hydroxide solution after a reaction ends to prepare finished products, wherein the molar ratio of the acetophenone to the hydrogen chloride ethanol solution is 1:1. The synthetic method is simple and convenient in process and high in product yield and is suitable for industrial production.
Owner:NANKAI UNIV +1
Preparation method of 2-(4-oxethyl phenyl)-2-methyl propanol
InactiveCN103467254AReduce riskReasonable workmanshipOrganic chemistryOrganic compound preparationPropanolPotassium borohydride
The invention provides a preparation method of 2-(4-oxethyl phenyl)-2-methyl propanol. The preparation method comprises the following steps of (1) dissolving 2-(4-oxethyl phenyl)-2-methyl ethyl propanoate in an ethyl alcohol solvent, and adding potassium borohydrate and lithium chloride; after ending reaction, dropwise adding hydrochloric acid, adding water, recovering the solvent under reduced pressure, extracting by using dichloromethane, drying an organic phase by using sodium sulphate anhydrous, and carrying out vacuum concentration, thus obtaining the 2-(4-oxethyl phenyl)-2-methyl propanol. The preparation method has the advantages that the dangerousness is reduced, and the process is relatively reasonable, simple and convenient. The 2-(4-oxethyl phenyl)-2-methyl propanol is low in cost, high in quality, environment-friendly and is suitable for industrial production. The reaction total yield can reach above 80% by the 2-(4-oxethyl phenyl)-2-methyl ethyl propanoate, and the content of the product reaches 98%.
Owner:LIANYUNGANG GUOSHENG CHEM
Method for synthesizing o-ethoxybenzoic acid from salicylic acid and acetone
ActiveCN103553909ALow costSimple processPreparation from carboxylic acid saltsOrganic compound preparationAcetic acidFiltration
The invention relates to a method for synthesizing o-ethoxybenzoic acid from salicylic acid and acetone, which comprises the following steps: adding salicylic acid, acetone and potassium hydroxide into a reaction bulb, dropwisely adding bromoethane while stirring at room temperature, heating under reflux for 9-15 hours, recovering the acetone under reduced pressure, adding water and liquid alkali, heating under reflux for 2-4 hours, cooling to room temperature, regulating the pH value to 3-4 with glacial acetic acid, cooling to 1-15 DEG C, separating by vacuum filtration, washing with water, and carrying out vacuum drying to obtain the o-ethoxybenzoic acid, wherein the salicylic acid:potassium hydroxide:bromoethane:liquid alkali mol ratio is 1:(2.0-2.5):(2.1-2.5):1.3. The method has the advantages of simple technique, low raw material cost, high yield and good quality, and is convenient to operate and convenient for industrial production.
Owner:苏州诚和医药化学有限公司
Citric acid alidenafil crystal form D and preparation method and usage thereof
The invention relates to 1-[3-(6, 7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazole parallel [4, 3-d] pyridine-5-thyl)-4-ethoxylbenzene sulfonyl]-cis-3, 5-lupetazin citrate or citric acid alidenafil crystal form D and preparation method thereof and also relates to drug combination containing citric acid alidenafil crystal form D and application thereof in preparation of drug for treating erection disturbance. Citric acid alidenafil raw material is dissolved in distilled water / acetone mixed solution in certain proportion, and heat preservation is carried out for 50-72 hours while stirring is carried out at certain temperature, thus obtaining the product. Test by an X-ray powder diffractometer, thermogravimetric analysis and infrared spectrometer proves that an unprecedented citric acid alidenafil crystal form D is obtained, the crystal form D can form a combination with one or multiple pharmaceutically acceptable carrier, excipient or diluent and can be effectively applied to treatment on andropathy.
Owner:刘桂坤
Crystal form V of Aildenafil citrate and preparation method and application thereof
InactiveCN101698668BGood chemical stabilitySuitable for long term storageOrganic active ingredientsOrganic chemistryAildenafilPharmaceutical Substances
The invention relates to a crystal form V of 1-[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H- pyrazolo[4,3-d] 5-pyrimidinyl)-4-ethyoxylbenzenesulfonyl]-cis-3,5-dimethylpiperazine citrate or Aildenafil citrate, and a preparation method thereof. The preparation method comprises the following steps: dissolving raw materials of the Aildenafil citrate in distilled water, stirring, and raising temperature to a reflux temperature; and adding acetone solution, and stirring and raising temperature continuously. The prepared product meets product requirements after being tested by an X-ray powder diffractometer and an infrared spectrometer. The invention also relates to a medicinal composition comprising the crystal form V of the Aildenafil citrate, and application of the crystal form V of the Aildenafil citrate in preparing medicaments for treating erection disturbance (ED).
Owner:刘桂坤
Aildenafil citrate crystal form O, preparation method and application thereof
InactiveCN101830903BSuitable for long term storageCrystal stableOrganic active ingredientsOrganic chemistry methodsDiseaseMethyl group
The invention relates to 1-[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazol[4,3-d] pyridine-5-group)-4-ethoxybenzene sulfonyl]-cis-3,5-lupetazin citrate or aildenafil citrate crystal form O and a preparation method thereof. The invention also relates to a medicine compound containing aildenafil citrate crystal form O and the application thereof in preparing the medicine for treating erectile dysfunction (ED). The aildenafil citrate crystal form O is formed by the steps of: solving citrate in the distilled water and tetrahydrofuran, stirring, rising temperature, filtering, stirring filtrate, decreasing temperature, crystallizing and filtering. The aildenafil citrate crystal form O is prepared into the medicine together with the medical excipients and is applied in treating the erectile dysfunction disease.
Owner:HONG KONG GOLDEN WISDOM MEDICAL PRODN LTD
Preparation method of bromine-containing azide
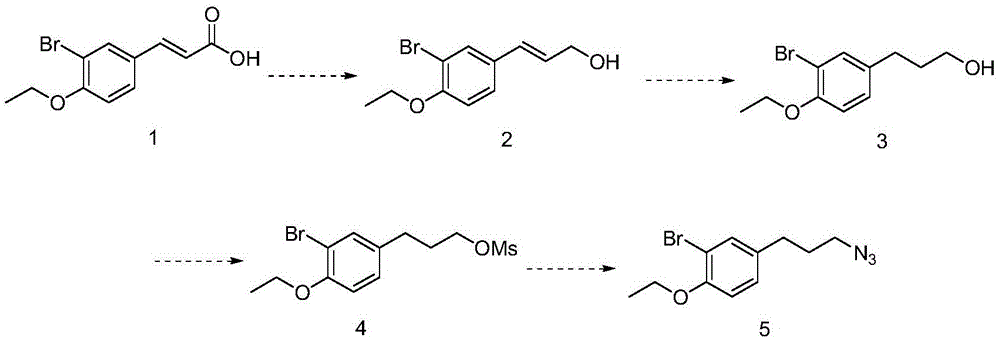
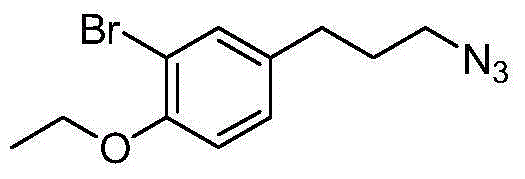
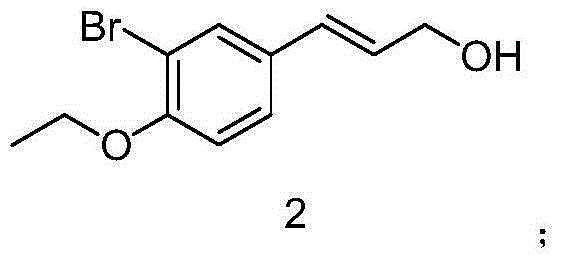
The invention discloses a preparation method of 3-bromophenyl azide1-(3-azidopropyl)-3-bromo-4-ethoxybenzene. 3-(3-bromo-4-ethoxyphenyl)acrylic acid is used as a starting material, a target product 5 is obtained through reduction, hydrogenation, Ms (methylsulfonyl) loading and azidation reactions, and the product is used as a template micro-molecule for synthesis of various compound libraries.
Owner:湖南华腾制药有限公司
O-ethoxyl phenol refining process
The o-ethoxyl phenol refining process includes the following steps: alkali washing condensated o-ethoxyl phenol oil phase or o-ethoxyl phenol product obtained through catechol process with 5-20 % sodium hydroxide solution for 30 min and separating liquid; regulating pH value of the water phase after alkali washing with hydrochloric acid to 1-4, extracting with toluene, and separating liquid to collect oil phase; distilling the oil phase at 110-120 deg.c to recover toluene, lowering the temperature to 30-40 deg.c for decompression rectification to obtain o-ethoxyl phenol of purity not lower than 99.2 %, with the recovered toluene being reused. The present invention can raise the production capacity of industrial apparatus and lower production cost.
Owner:CNPC JILIN CHEM GROUP CORP
Method for synthesizing furan phenol by using aluminum dicarboxylate phenol as catalyst
InactiveCN101979387AEasy to manufactureEasy to useOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsFuranEconomic benefits
The invention discloses a method for synthesizing furan phenol by using aluminum dicarboxylate phenol as a catalyst, which is to synthesize the furan phenol (1) under the catalytic action of the aluminum dicarboxylate phenol by using 2-(2-methylallyloxy)phenol as a raw material. The reaction equation is as follows: (RCO2)2AlOAr, wherein R may be C1 to C2 alkyl or C3 to C17 linear alkyl, branched alkyl or cycloalkyl; Ar may be phenyl, 2-methylphenyl, 4-methylphenyl, 2-methoxyphenyl, 4-methoxyphenyl, 2-ethyoxylphenyl, 4-ethyoxylphenyl, 2-phlorphenyl, 4-phlorphenyl, 2-carboxylphenyl, 2,6-dimethylphenyl, 2-biphenyl, 4-biphenyl, 1-naphthyl and 2-naphthyl. In the invention, furan phenol is synthesized by using aluminum dicarboxylate phenol as the catalyst, the raw material is readily available, production devices are not changed, the investment is small and quick in effectiveness, the furan phenol cyclization single-step yield is more than or equal to 81.0 percent, the furan phenol yield is much higher than that of the traditional process which uses aluminium isopropoxide as the catalyst, the production cost is reduced, and the economic benefit is obvious.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com