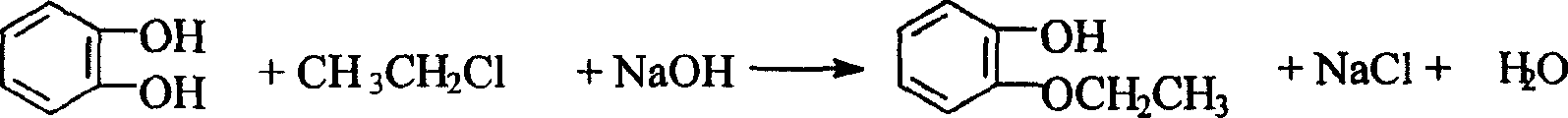O-ethoxyl phenol synthesizing process
A technology of o-ethoxyphenol and synthesis method, applied in the direction of organic chemistry, can solve the problems of high yield, high toxicity of diethyl sulfate, serious environmental pollution, etc., and achieve short process, low one-time investment and low product cost Falling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In the autoclave, add toluene 800g, water 480g, start stirring, turn on the autoclave shaft cooling water, then add catechol 176g, sodium carbonate 8g, tetrabutylammonium bromide 12g, solid sodium hydroxide 89.6g, chlorine 165g of ethane, after sealing the cover, fill it with nitrogen to 1.0Mpa, keep it for 2 minutes, check for air leakage, if there is no air leakage, release the pressure. Start to heat up, keep at 130-139°C and 0.1-1.1MPa pressure for 2 hours, cool down to room temperature after the reaction, open the autoclave after pressure relief, pour the feed liquid into the separatory funnel, and separate the liquids. Add hydrochloric acid to the water phase to adjust the pH value to 5-6. After fully extracting with toluene, combine the oil phases. The oil layer is distilled under normal pressure and under reduced pressure to obtain o-ethoxyphenol products with a purity ≥ 98.0%. The solvent recovered by distillation is recycled. The obtained product was weighed...
Embodiment 2
[0031] In the autoclave, add 800g of toluene, 500g of water, start stirring, turn on the cooling water of the autoclave shaft, then add 220g of catechol, 20g of sodium carbonate, 9g of tetraethylammonium bromide, 104g of solid alkali, and 180g of ethyl chloride , After sealing the cover, fill nitrogen to 1.0Mpa, keep it for 2 minutes, check for air leakage, if there is no air leakage, release the pressure. Start to heat up, keep at 120-130°C and 0.1-1.1MPa pressure for 4 hours, cool down to room temperature after the reaction is over, open the autoclave after pressure relief, pour the feed liquid into the separatory funnel, and separate the liquids. Add hydrochloric acid to the water phase to adjust the pH value to 5-6. After fully extracting with toluene, combine the oil phases. The oil layer is distilled under normal pressure and under reduced pressure to obtain o-ethoxyphenol products with a purity ≥ 98.0%. The solvent recovered by distillation is recycled. The obtained p...
Embodiment 3
[0033] In the autoclave, add 500g of toluene, 350g of water, start stirring, turn on the cooling water of the autoclave shaft, then add 220g of catechol, 60g of sodium carbonate, 2g of tetramethylammonium bromide, 80g of solid alkali, and 130g of ethyl chloride , After sealing the cover, fill nitrogen to 1.0Mpa, keep it for 2 minutes, check for air leakage, if there is no air leakage, release the pressure. Start to heat up, keep at 100-120°C and 0.2-1.0MPa pressure for 6 hours, cool down to room temperature after the reaction is over, open the autoclave after the pressure is released, pour the feed liquid into the separatory funnel, and separate the liquids. Add hydrochloric acid to the water phase to adjust the pH value to 5-6. After fully extracting with toluene, combine the oil phases. The oil layer is distilled under normal pressure and under reduced pressure to obtain o-ethoxyphenol products with a purity ≥ 98.0%. The solvent recovered by distillation is recycled. The o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com