Preparation method and application of fluorine-containing microspheres without stabilizers on surfaces
A stabilizer and microsphere technology, used in coatings, powder coatings, etc., can solve problems such as harming the health of construction workers, cumbersome processing, and a large amount of organic solvents, achieving good hydrophobicity and corrosion resistance, simple preparation method, Fast response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
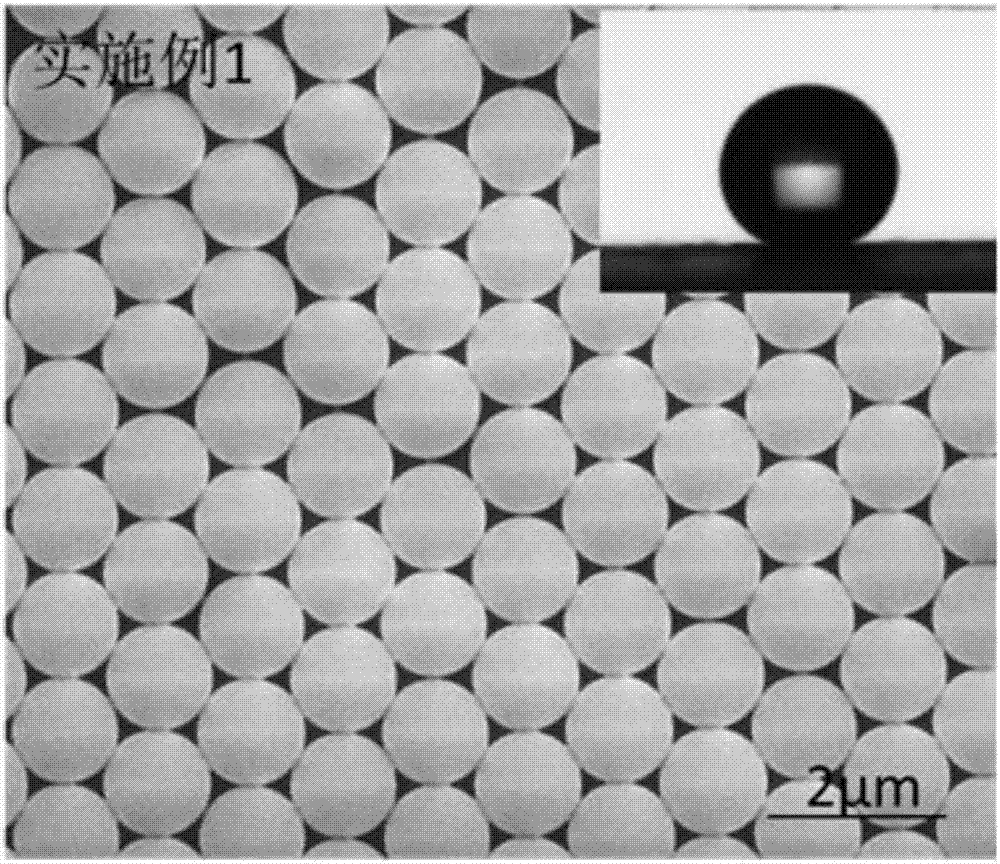
Embodiment 1
[0052] 1) Add 120 mL of anhydrous tetrahydrofuran (THF) to a single-necked flask, add 2 mmol of polyethylene glycol monomethyl ether (mPEG), 4 mmol of RAFT reagent 4-(2-carboxyethylcarbonyl) oxyethoxyphenyl di Isobutyronitrile thiocarbamate (CEPDB) and 0.2 mmol 4-dimethylaminopyridine (DMAP) were dissolved in anhydrous tetrahydrofuran (THF), and the one-necked flask was placed in an ice-water bath and cooled to 0°C with stirring;
[0053] 2), 2), 4mmol dicyclohexylcarbodiimide (DCC) was dissolved in 30mL anhydrous tetrahydrofuran (THF) to obtain a DCC-THF solution, and the DCC-THF solution was added dropwise in a single-necked flask, and the dropping time was controlled at After 30 minutes of dripping, continue to place the reaction kettle in an ice-water bath for 1 hour after the addition;
[0054] 3), the one-necked flask was taken out from the ice-water bath, then stirred and reacted at room temperature for 48 hours, and the insoluble matter dicyclohexyl urea (DCU) in the r...
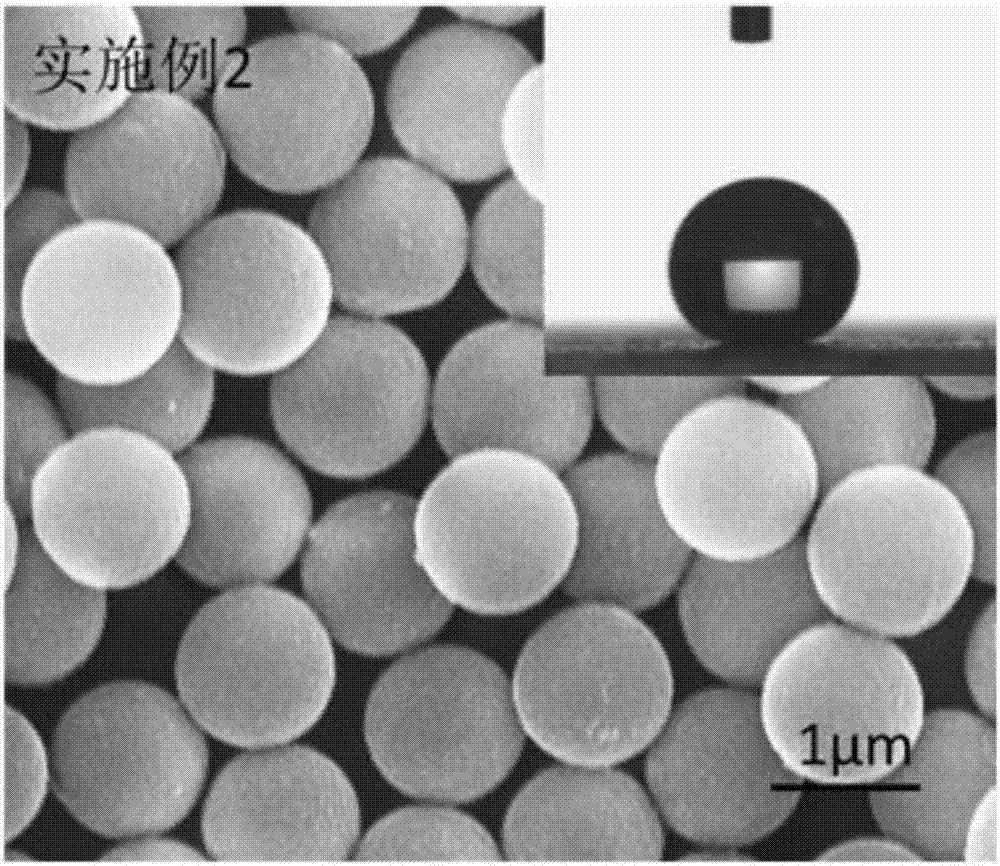
Embodiment 2
[0069] 1) Add 120 mL of anhydrous tetrahydrofuran (THF) to a single-necked flask, add 2 mmol of polyethylene glycol monomethyl ether (mPEG), 2.2 mmol of RAFT reagent 4-(2-carboxyethylcarbonyl)oxyethoxyphenyl Isobutyronitrile dithiocarbamate (CEPDB) and 0.02 mmol 4-dimethylaminopyridine (DMAP) were dissolved in anhydrous tetrahydrofuran (THF), and the one-necked flask was placed in an ice-water bath and cooled to 0°C with stirring;
[0070] 2), 2), 2mmol dicyclohexylcarbodiimide (DCC) was dissolved in 30mL anhydrous tetrahydrofuran (THF) to obtain a DCC-THF solution, and the DCC-THF solution was added dropwise in a single-necked flask, and the dropping time was controlled at After 40 minutes of dripping, continue to place the reaction kettle in an ice-water bath for 1 hour after the addition;
[0071] 3), the one-necked flask was taken out from the ice-water bath, then stirred and reacted at room temperature for 48 hours, and the insoluble matter dicyclohexyl urea (DCU) in the ...
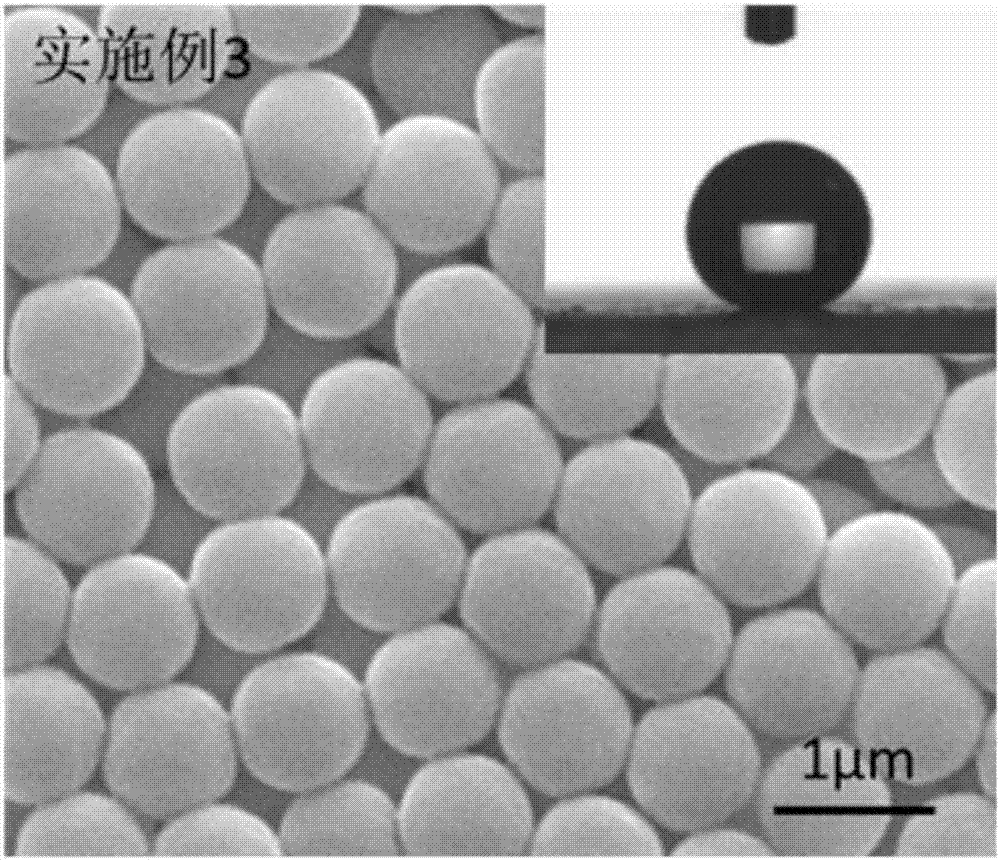
Embodiment 3
[0086] 1) Add 120 mL of anhydrous tetrahydrofuran (THF) to a single-necked flask, add 2 mmol of polyethylene glycol monomethyl ether (mPEG), 3 mmol of RAFT reagent 4-(2-carboxyethylcarbonyl) oxyethoxyphenyl di Isobutyronitrile thiocarbamate (CEPDB) and 0.1 mmol 4-dimethylaminopyridine (DMAP) were dissolved in anhydrous tetrahydrofuran (THF), and the one-necked flask was placed in an ice-water bath and cooled to 0°C with stirring;
[0087] 2) Dissolve 5mmol of dicyclohexylcarbodiimide (DCC) in 30mL of anhydrous tetrahydrofuran (THF) to obtain a DCC-THF solution, add the DCC-THF solution dropwise into a single-necked flask, and the dropping time is controlled within 30 minutes to complete the drop After the dropwise addition is completed, continue to place the reaction kettle in an ice-water bath for 1 hour of reaction;
[0088] 3), the one-necked flask was taken out from the ice-water bath, then stirred and reacted at room temperature for 48 hours, and the insoluble matter dicy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com