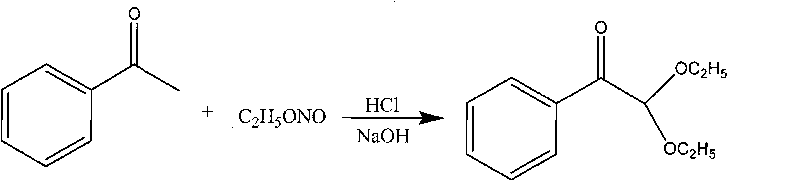
Synthetic method of 2,2-diethoxy acetophenone photoinitiator
A technology of diethoxyacetophenone and photoinitiator, which is applied in the field of photoinitiator 2, can solve the problems of high corrosion of hydrogen chloride gas, difficulty of industrial production, inconvenience of industrial production, etc., and achieves no impact on the environment and is suitable for industrial production , Raw and auxiliary materials are cheap and easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Step a, synthetic ethyl nitrite
[0018] Set a four-necked reaction flask equipped with mechanical stirring and air duct, add 75 grams of sodium nitrite, 46 grams of absolute ethanol and 190 grams of water, add dropwise 270 grams of 20% dilute sulfuric acid at room temperature, and generate ethyl nitrite gas Directly used in step ii reaction.
[0019] Step b, synthetic 2,2-diethoxyacetophenone
[0020] A four-necked reaction flask equipped with mechanical stirring, a thermometer and a gas inlet tube was installed, and 60 grams of acetophenone and a solution mixed with 50 grams of 36% hydrogen chloride ethanol solution and 300 grams of absolute ethanol were added, cooled to 5 ° C, Introduce the ethyl nitrite generated in step i, and continue to incubate at 40° C. for 3 hours after aeration is completed. Ethanol was distilled off under reduced pressure, and the raffinate was neutralized to neutrality with 20% mass concentration of sodium hydroxide solution, then extract...
Embodiment 2
[0022] Step a, synthetic ethyl nitrite
[0023] Set up a reaction kettle equipped with mechanical stirring and air guide port, add 150 kg of sodium nitrite, 92 kg of absolute ethanol and 380 kg of water, and drop 540 kg of 20% dilute sulfuric acid at 25°C to produce ethyl nitrite gas For the reaction of step ii.
[0024] Step b, synthetic 2,2-diethoxyacetophenone
[0025] A reaction kettle equipped with mechanical stirring and gas inlet pipe is set, add 120 kilograms of acetophenone, 100 kilograms of 36% hydrogen chloride ethanol solution and 500 kilograms of dehydrated alcohol, cool to 5 ℃, pass into the ethyl nitrite that step i generates gas, react at 40°C for 3 hours after ventilation is completed, distill ethanol off under reduced pressure, neutralize the residual liquid with 240 kg of 20% mass concentration of sodium hydroxide solution, extract with anhydrous ether, dry over anhydrous sodium sulfate, filter, and remove The crude product was dissolved, and 148 kg of 2,2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com