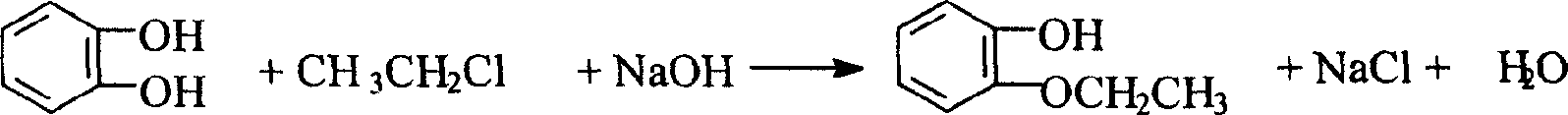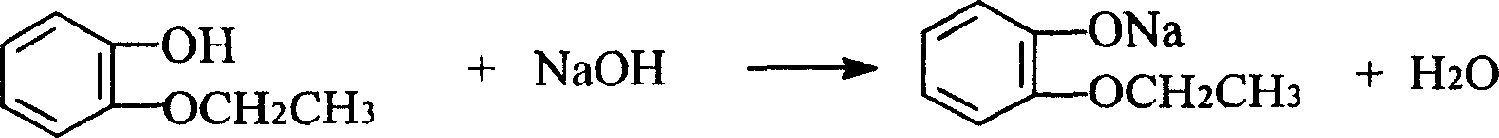O-ethoxyl phenol refining process
A technology for o-ethoxyphenol and ethoxyphenol, which is applied in the refining field of synthesizing o-ethoxyphenol by a catechol method, can solve problems such as being difficult to separate, achieve high product purity, reduce production costs, and improve The effect of productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Take 1090g of the above-mentioned condensed liquid oil phase, divide it into two equal parts, and determine it as the first and second batches, and carry out the following steps respectively: wash with 960g of 5% sodium hydroxide solution for 30 minutes, and then separate the liquids; Add 31% hydrochloric acid to neutralize until the pH value is 1, then add 600 g of toluene to fully extract, separate the layers, and collect the oil phase. Then add the extracted oil phase into the distillation flask, install the condenser, tail pipe, receiver and thermometer, and heat to 140°C with an oil bath for distillation. When the column temperature is between 110°C and 120°C, distillates begin to flow out from the top of the column, and the effluent at this time is mainly toluene and contains a small amount of water. When the toluene is almost evaporated, the column temperature begins to drop, and when the column temperature is 30°C to 40°C, the atmospheric distillation ends. Aft...
Embodiment 2
[0030] Take 1090g of the above-mentioned condensed liquid oil phase, divide it into two equal parts, and determine it as the 3rd and 4th batches, and carry out the following steps respectively: wash with 480g of 10% sodium hydroxide solution for 30 minutes, and then separate the liquids; Add 31% hydrochloric acid to neutralize until the pH value is 2, then add 600 g of toluene to fully extract and separate the layers. Collect the oily phase. Then add the extracted oil phase into the distillation flask, install the condenser, tail pipe, receiver and thermometer, and heat to 140°C with an oil bath for distillation. When the column temperature is between 110°C and 120°C, distillates begin to flow out from the top of the column, and the effluent at this time is mainly toluene and contains a small amount of water. When the toluene is almost evaporated, the column temperature begins to drop, and when the column temperature is 30°C to 40°C, the atmospheric distillation ends. After ...
Embodiment 3
[0032] Take 1090g of the above-mentioned condensed liquid oil phase, divide it into two equal parts, and determine it as the 5th and 6th batches, and carry out the following steps respectively: wash with 20% sodium hydroxide solution 480g alkali for 30 minutes, then separate the liquids; take the water after alkali washing Add 31% hydrochloric acid to neutralize until the pH value is 4, then add 600 g of toluene to fully extract and separate the layers. Collect the oily phase. Then add the extracted oil phase into the distillation flask, install the condenser, tail pipe, receiver and thermometer, and heat to 140°C with an oil bath for distillation. When the column temperature is between 110°C and 120°C, distillates begin to flow out from the top of the column, and the effluent at this time is mainly toluene and contains a small amount of water. When the toluene is almost evaporated, the column temperature begins to drop, and when the column temperature is 30°C to 40°C, the at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com