Thorium-containing fluoride fused salt and/or uranium-containing fluoride fused salt and preparation method
A fluoride and molten salt technology, which is applied in the fields of reactor fuel material, nuclear power generation, climate sustainability, etc., can solve the problem that thorium and/or uranium elements cannot be uniformly distributed in fluoride molten salt, etc., and achieves a simple and easy-to-operate preparation method. , the effect of not easy to reunite
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6
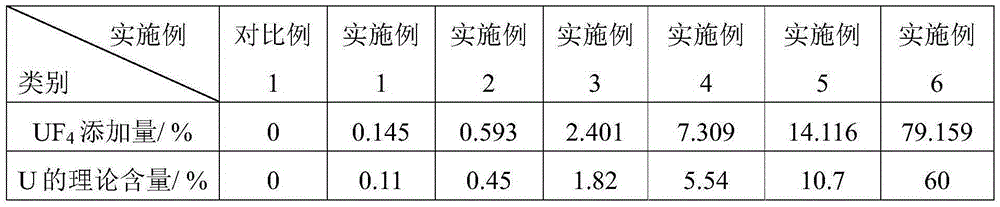
[0031] The product that embodiment 1~6 makes is FLiNaKU molten salt, and its difference is only in UF 4 The addition amount is different, UF in the embodiment 1~6 4 The amount of addition is as described in Table 1.
[0032] Table 1
[0033]
[0034] Note: UF 4 The percentages in the added amount of U are all mass percentages relative to the total amount of LiF, NaF and KF; the percentages in the theoretical content of U are all mass percentages relative to the total amount of the sample.
[0035] The preparation method of FLiNaKU molten salt in embodiment 1~6 all comprises the following steps:
[0036] Using the ball mill crushing and mixing method, weigh 15g of three kinds of fluorides LiF, NaF and KF according to the mass ratio of 29.3:11.7:59 and mix them into the ball mill. After grinding for 3 hours, the particle size is 0.2 ~ 1μm; 1 in UF 4 The raw materials were put into the ball mill respectively, and after being ground by the ball mill for 3 hours, the partic...
Embodiment 7~12
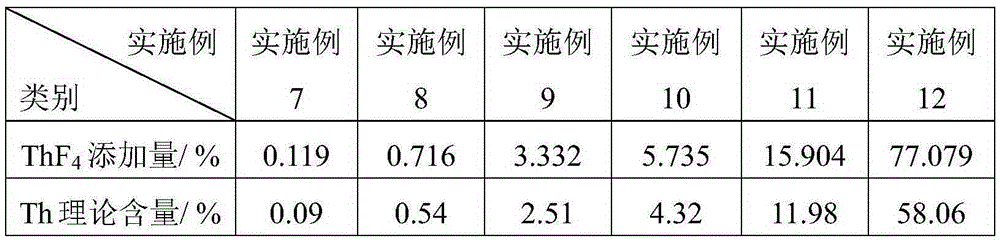
[0040] The products obtained in Examples 7-12 are all FLiNaKTh molten salts, and the difference is only that ThF 4 The amount of addition is different, ThF in Examples 7-12 4 The amount of addition is as described in Table 2.
[0041] Table 2
[0042]
[0043] Note: ThF 4The percentages in the added amount of Th are all mass percentages relative to the total amount of LiF, NaF and KF; the percentages in the theoretical content of Th are all mass percentages relative to the total amount of the sample.
[0044] The preparation method of FLiNaKTh molten salt in embodiment 7~12, compared with embodiment 1~6, except UF 4 Replaced by ThF 4 Except, other operations or conditions are all the same as in Examples 1-6.
Embodiment 13~16
[0046] The products obtained in Examples 13 to 16 are all mixed homogeneous molten salts containing Th and U, and the only difference is that UF 4 and ThF 4 The addition amount is different, UF in the embodiment 13~16 4 and ThF 4 The amount of addition is as described in Table 3.
[0047] table 3
[0048]
[0049]
[0050] Note: UF 4 and ThF 4 The percentages in the added amount are all mass percentages relative to the total amount of LiF, NaF and KF; the percentages in the theoretical content of Th and U are all mass percentages relative to the total amount of the sample.
[0051] The preparation method of molten salt in embodiment 13~16, compared with embodiment 1~6, except UF 4 Replaced by UF 4 and ThF 4 Except the mixture, all the other operations or conditions are the same as in Examples 1-6.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com