Serum-free medium for inducing differentiation of umbilical cord mesenchymal stem cells into insulin secretory-like cells and preparation method and application thereof
A serum-free medium, insulin secretion technology, applied in cell culture active agents, biochemical equipment and methods, animal cells, etc., can solve the problems of prohibitive cost and limited number of organs, achieve high-efficiency solutions, and avoid cell growth process. Instability, the effect of eliminating the risk of spreading xenogeneic pathogens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Screening of medium composition
[0061] Basal medium: DMEM (glucose content 4.5g / L)+5%SR+1%NEAA+2%B27(50X)+1%N-2(100X)+10ng / ml HGF+10ng / ml EGF+10ng / ml b-FGF+10mmol / L Nicotinamide;
[0062] Screening components: add 100ng / ml vinblastine III (Conophylline), 1 volume part of ITS, 1ug / ml heparin (Heparin), and 10ng / ml betacellulin (betacellulin) to the basal medium ), sialidin 4 (Exendi-4), insulin-like growth factor (IGF-1), and pentagastrin. The groupings are listed in Table 3 below.
[0063] table 3
[0064]
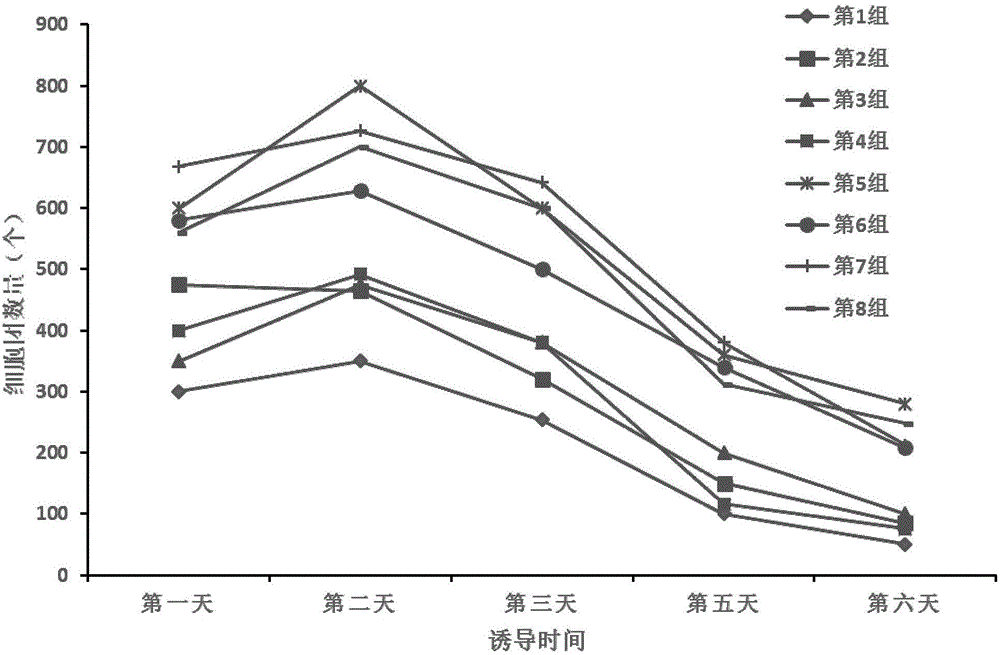
[0065] In a biological safety cabinet, the third-generation hUC-MSCs isolated from the Wharton's glue tissue of the umbilical cord of spontaneously delivered neonates were collected at 5 × 10 4 cells / cm 2 The density was inoculated into the ultra-low adsorption six-well plate, and 2 ml of the test medium shown in Table 3 was added to each well to observe the cell growth and the number of cell clusters.
[0066] Results: In the first group of ...
Embodiment 2
[0067] Example 2 Screening of the Content of Medium Components (Vinblastine Ⅲ)
[0068] Test medium: 89 parts by volume of high glucose DMEM (glucose content 4.5g / L), 5 parts by volume of serum replacement (SR), 2 parts by volume of B27 (50X), 1 part by volume of ITS, 1 part by volume of non-essential amino acids (NEAA), 1 volume of N-2 (100X), heparin (Heparin) at a final concentration of 1ug / ml, nicotinamide at a final concentration of 10mmol / L, recombinant human alkaloids at a final concentration of 10ng / ml Fibroblast growth factor (b-FGF), epidermal growth factor (EGF), hepatocyte growth factor (HGF), pentagastrin and concentration gradients of 1, 10, 50, 100, 200, 300 ng / ml Vinblastine III (Conophylline).
[0069] Human umbilical cord mesenchymal stem cells were induced to differentiate and cultured using the above-mentioned media with different concentrations of vinblastine III (Conophylline).
[0070] RESULTS: In the two groups of test media with the concentration o...
Embodiment 3
[0071] Example 3. Screening of the content of medium components (pentagastrin)
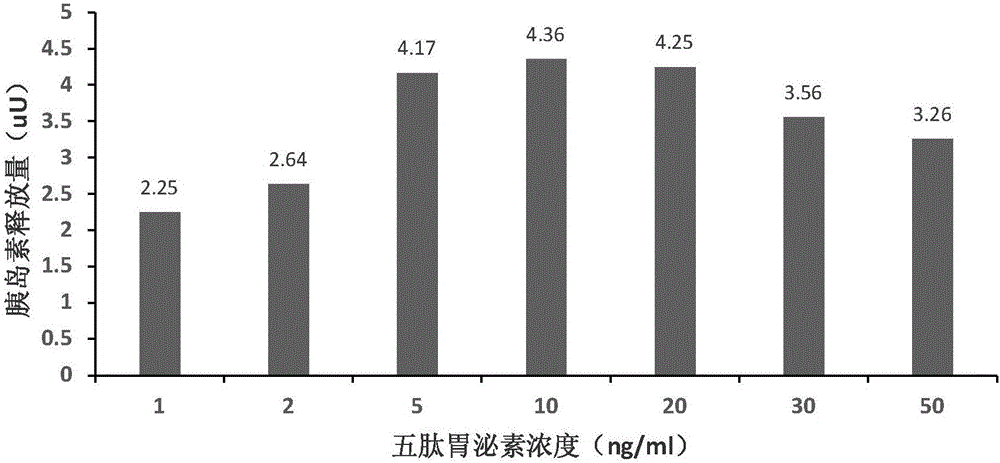
[0072] Test medium: 89 parts by volume of high glucose DMEM (glucose content 4.5g / L), 5 parts by volume of serum replacement (SR), 2 parts by volume of B27 (50X), 1 part by volume of ITS, 1 part by volume of non-essential amino acids (NEAA), final concentration of 1ug / ml heparin (Heparin), 1 volume of N-2 (100X), 100ng / ml vinblastine III (Conophylline), 10mmol / L nicotinamide, Recombinant human basic fibroblast growth factor (b-FGF), epidermal growth factor (EGF), hepatocyte growth factor (HGF) with a final concentration of 10ng / ml and a concentration gradient of 1, 2, 5, 10, 20 , 30, 50ng / ml of pentagastrin.
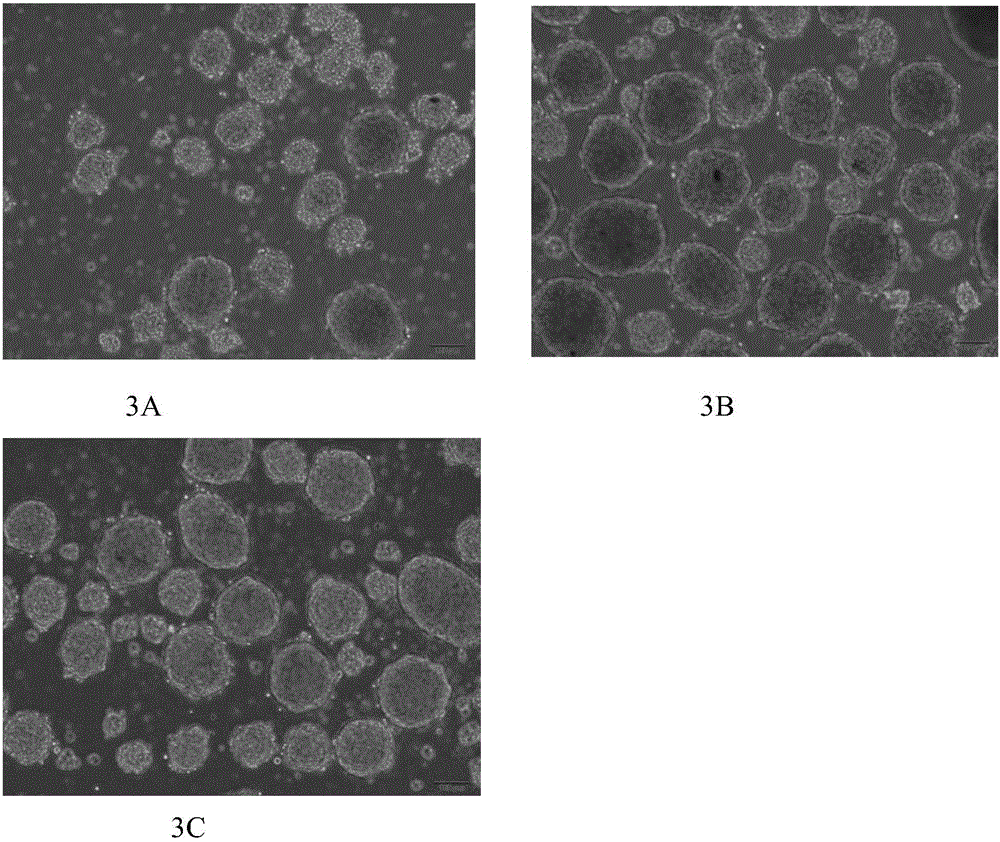
[0073] The above-mentioned media with different concentrations of pentagastrin were used to induce differentiation and culture of human umbilical cord mesenchymal stem cells. 4 A) the amount of insulin released in each group, and the cells in each group were compared. The sugar stimulat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com