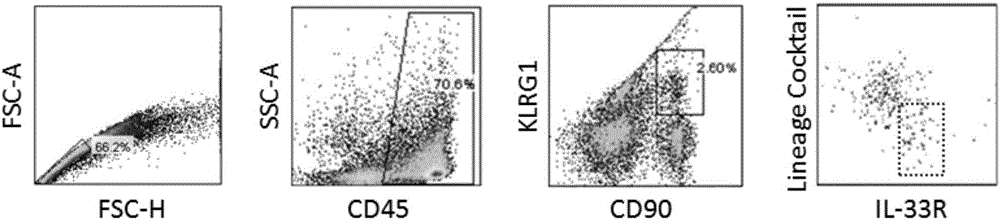
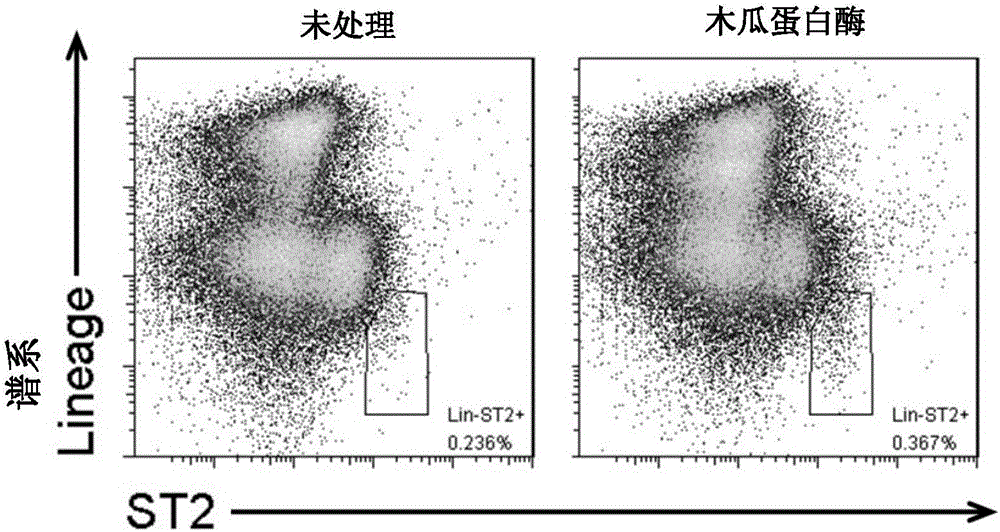
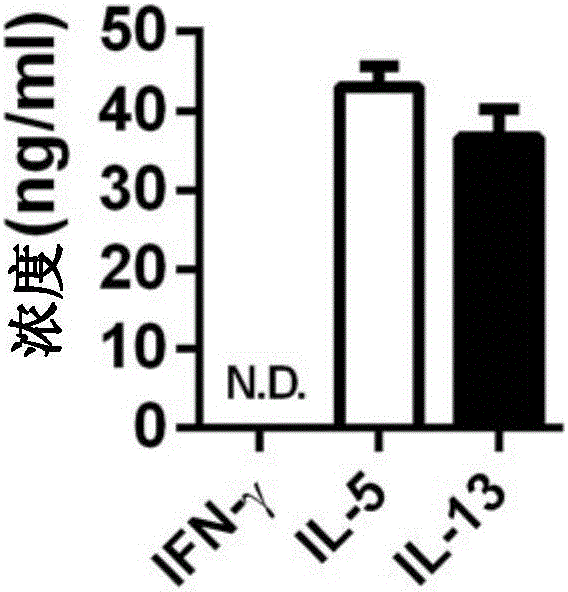
Method of separating and purifying II-type innate lymphoid cells from animal lung tissue
A lymphocyte, separation and purification technology, applied in animal cells, cell dissociation methods, vertebrate cells, etc., can solve the problems of small number of ILC2 cells, lack of cell surface, and no description of the isolation method of mouse lung ILC2 cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The following experiments require aseptic technique.
[0034] (1) Normal or mouse lung tissue with lung inflammation was placed in sterile PBS and placed on ice.
[0035] (2) Cut the tissue into small pieces of about 1-2mm3, put the tissue piece into a centrifuge tube, add 5ml of collagenase I solution (0.1%, 1mg / ml, sigma) prepared by incomplete DM EM, tighten the cap and place Digest for 30 minutes at 220 rpm in a shaking box at 37°C (it can be seen that the tissue block has dispersed and lost the shape of the block, that is, the digestion is successful).
[0036] (3) After step 3 is completed, put it on ice to stop digestion, filter through a 200-mesh sieve, and gently grind the lung tissue remaining on the net before filtering.
[0037] (4) Centrifuge at 1060g at 4°C for 10 minutes, discard the supernatant. (The following centrifugation is at 4°C except that the step of density gradient centrifugation is at room temperature).
[0038] (5) Density gradient centrif...
Embodiment 2
[0052] The lungs of normal mice were digested with several different concentrations of collagenase I solutions prepared with physiological saline by the method of the present invention, and other operations were the same as in Example 1. The results showed that highly active type II innate lymphocytes could be isolated and purified. For the number of type II innate lymphocytes that can be obtained see Figure 4A .
[0053] After the lung inflammation was induced by 80 micrograms of blemisin intranasally in normal mice for one week, type II innate lymphocytes were isolated according to the method of Example 1. The results showed that highly active type II innate lymphocytes could be isolated and purified, and the resulting II Type innate lymphocyte count see Figure 4B .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com