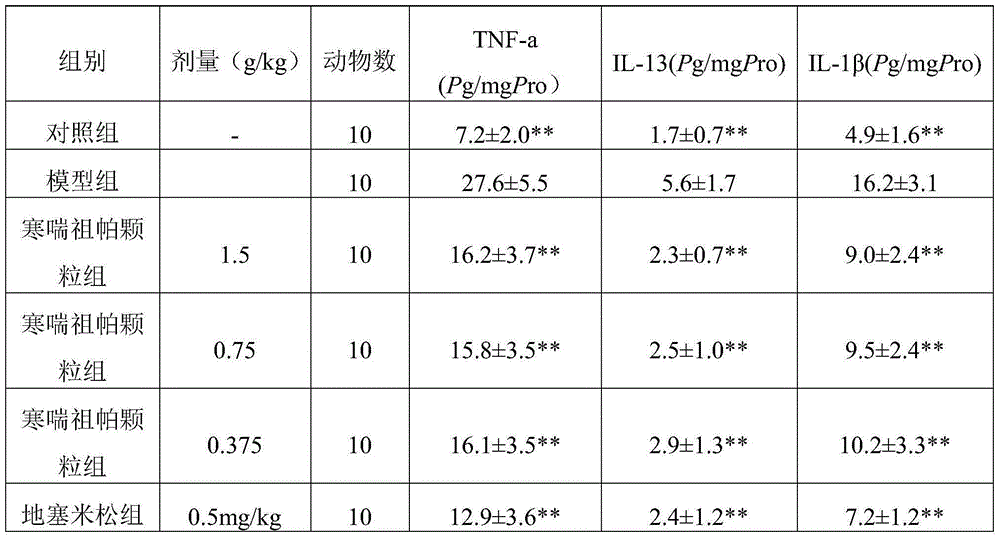
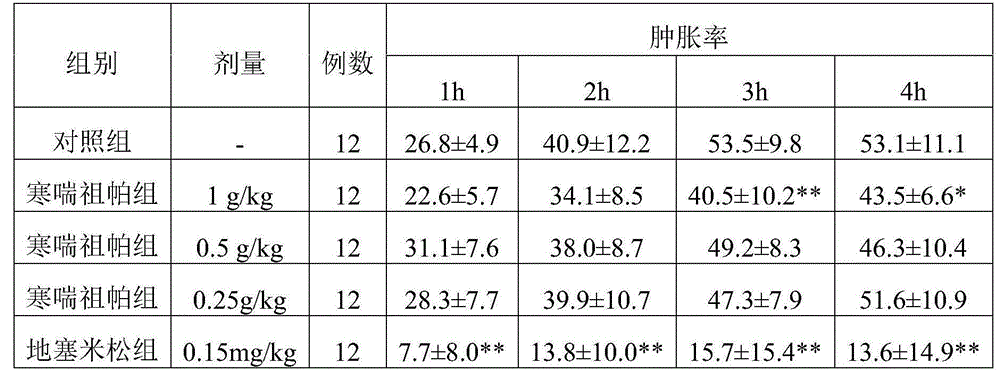
New medical use of Hanchuan zupa granules
A zupa and granule technology, applied in the field of new medical uses of Hanchuan zupa granules, can solve the problems of acute lung injury and inflammation of "Hanchuan zupa granules".
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 medicine of the present invention
[0034] Prescription: 150 parts by weight of fennel, 150 parts by weight of celery seeds, 90 parts by weight of fenugreek, 90 parts by weight of rose, 90 parts by weight of rue, 80 parts by weight of nettle seeds, 90 parts by weight of maidenhair fern, 90 parts by weight of fenugreek, 80 parts by weight of licorice extract.
[0035] Preparation method: Weigh each raw material according to the prescription amount, except the licorice extract, which is crushed into fine powder, and the other 8 raw materials such as fennel, decoct with water for 3 times, combine the decoction, filter, and concentrate the obtained filtrate to a clear paste with a relative density of 1.32 to 1.35, and mix the obtained clear paste with the fine powder of licorice extract, add sucrose and dextrin to the obtained mixture extract, and granulate the obtained mixture to obtain Granules.
Embodiment 2
[0036] The preparation of embodiment 2 medicines of the present invention
[0037] Prescription: 125 parts by weight of cumin, 125 parts by weight of celery seeds, 75 parts by weight of fenugreek, 75 parts by weight of rose, 75 parts by weight of rue, 70 parts by weight of nettle seeds, 75 parts by weight of maidenhair fern, 75 parts by weight of fenugreek part, 70 parts by weight of licorice extract.
[0038]Preparation method: Weigh each raw material according to the prescription amount, except the licorice extract, which is crushed into fine powder, and the other 8 kinds of raw materials such as fennel, decoct with water for 3 times, combine the decoction, filter, and concentrate the filtrate to a relatively high temperature. The clear paste with a density of 1.32-1.35 is obtained by mixing the obtained clear paste with the fine powder of licorice extract, drying the obtained mixture in turn, and pulverizing.
Embodiment 3
[0039] Embodiment 3 preparation of medicine of the present invention
[0040] Prescription: 100 parts by weight of fennel, 200 parts by weight of celery seeds, 85 parts by weight of celery, 85 parts by weight of rose, 85 parts by weight of rue, 60 parts by weight of nettle seeds, 85 parts by weight of maidenhair fern, 85 parts by weight of fenugreek, 60 parts by weight of licorice extract.
[0041] Preparation method: Weigh each raw material according to the prescription amount, except the licorice extract, which is crushed into fine powder, and the other 8 kinds of raw materials such as fennel, decoct with water for 3 times, combine the decoction, filter, and concentrate the filtrate to a relatively high temperature. Clear paste with a density of 1.32 to 1.35, and mix the obtained clear paste with fine powder of licorice extract, then add sucrose and dextrin to the obtained mixture extract, and granulate the obtained mixture to obtain granules agent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com