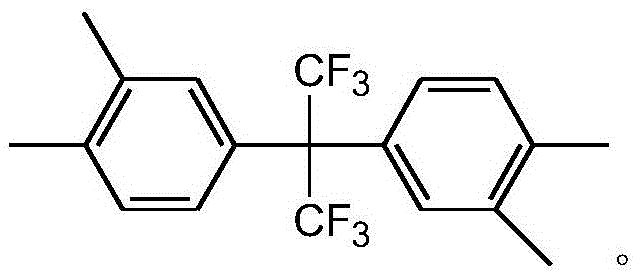
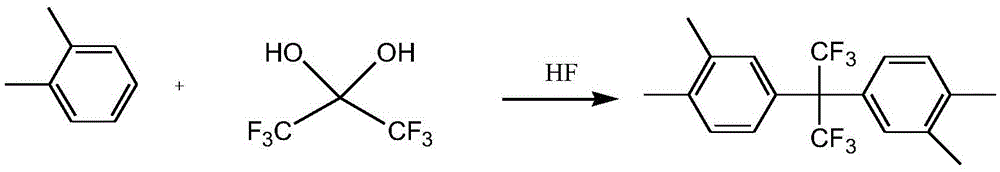
Preparation method of 2,2-bis(3,4-dimethylphenyl)hexafluoropropane
A technology of hexafluoropropane and xylyl, which is applied in the field of preparation of 2,2-dihexafluoropropane, which can solve the problems of strong toxicity and difficult transportation of hexafluoroacetone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In a 500mL three-neck flask equipped with magnetic stirring, condenser, oil bath heating, and water separator, add 82g (0.372mol) of hexafluoroacetone trihydrate and 80g (0.754mol) of o-xylene, layer, stir, and react The liquid is a milky white mixture, warming up to the reflux temperature, water is separated out in the water separator (about 40g o-xylene is added in the water separator), and the heating is continued to reflux until basically no water is released, and the liquid in the reaction bottle is colorless and transparent, which is o-xylene. Mixture of xylene and hexafluoroacetone monohydrate; middle and lower layer of water trap 15g, 11.9mL, density 1.26g / cm 3 , a small amount of hexafluoroacetone trihydrate separated out.
[0025] Add the mixture of o-xylene and hexafluoroacetone monohydrate obtained above into a 0.5L autoclave, add 127g (6.35mol) of hydrofluoric acid, start stirring, raise the temperature to 115°C for about 17h, and the reaction pressure is a...
Embodiment 2
[0028] In a 500mL three-neck flask equipped with magnetic stirring, condenser, oil bath heating, and water separator, add 82g (0.372mol) of hexafluoroacetone trihydrate and 80g (0.754mol) of o-xylene, layer, stir, and react The liquid is a milky white mixture, warming up to the reflux temperature, water is separated out in the water separator (about 40g o-xylene is added in the water separator), and the heating is continued to reflux until basically no water is released, and the liquid in the reaction bottle is colorless and transparent, which is o-xylene. Mixture of xylene and hexafluoroacetone monohydrate; middle and lower layer of water trap 15g, 11.9mL, density 1.26g / cm 3 , a small amount of hexafluoroacetone trihydrate separated out.
[0029] Add the mixture of o-xylene and hexafluoroacetone monohydrate obtained above into a 0.5L autoclave, add 150g (7.5mol) of hydrogen fluoride and hydrofluoric acid, start stirring, raise the temperature to 125°C for about 25 hours, and ...
Embodiment 3
[0032] In a 500mL three-neck flask equipped with magnetic stirring, condenser, oil bath heating, and water separator, add 82g (0.372mol) of hexafluoroacetone trihydrate and 90g (0.85mol) of o-xylene, layer, stir, and react The liquid is a milky white mixture, warming up to the reflux temperature, water is separated out in the water separator (about 40g o-xylene is added in the water separator), and the heating is continued to reflux until basically no water is released, and the liquid in the reaction bottle is colorless and transparent, which is o-xylene. Mixture of xylene and hexafluoroacetone monohydrate; middle and lower layer of water separator 16g, 12.3mL, density 1.30g / cm 3 , a small amount of hexafluoroacetone trihydrate separated out.
[0033] Add the mixture of o-xylene and hexafluoroacetone monohydrate obtained above into a 0.5L autoclave, add 111g (5.58mol) of hydrogen fluoride and hydrofluoric acid, start stirring, raise the temperature to 100°C for about 13 hours,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com