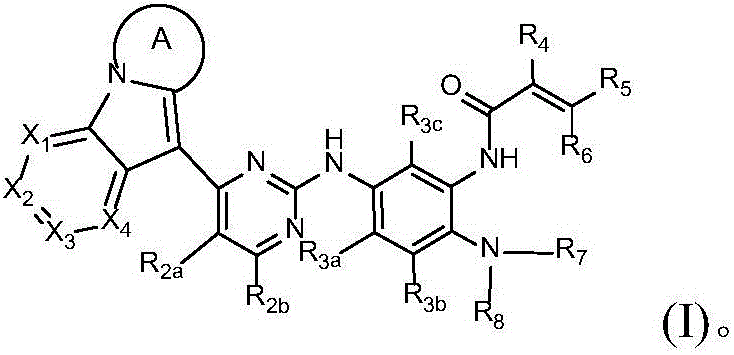
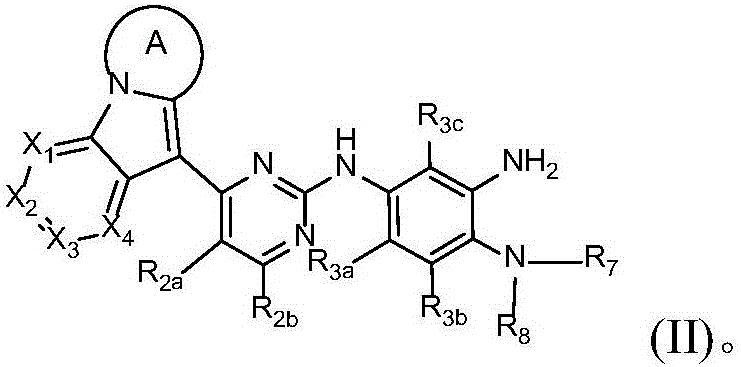
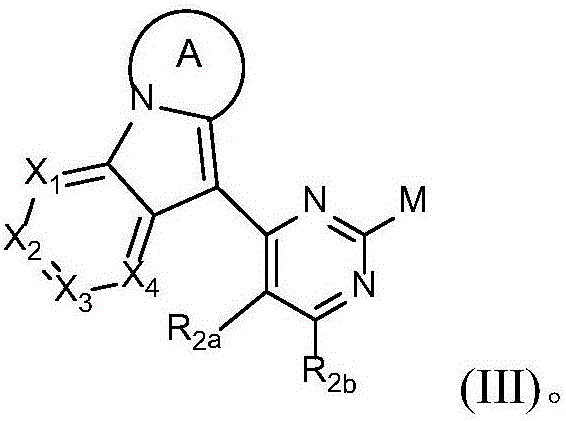
Novel epidermal growth factor receptor inhibitor and application thereof
A technology of atoms and isomers, applied in the field of cancer therapeutic drugs, can solve problems affecting patient compliance, occupying a large space, and not being able to effectively block EGFR activation signals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] Example 1 N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(2,3-dihydro-1H- Synthesis of pyrrolo[1,2-a]-indol-9-yl)pyrimidin-2-yl)amino)phenyl)acrylamide
[0160]
[0161] Step a Synthesis of 1-(3-bromopropyl)-1H-indole
[0162]
[0163] In a 100ml reaction flask, add NaH (60% content, 1.23g, 30.73mmol) and DMF (10ml) in turn, stir at room temperature for 5min and cool to 0-4°C, slowly add 10mL of dissolved indole (3g, 25.61mmol) DMF solution, after the addition was completed, raised to room temperature and reacted for 20 minutes to obtain an indole activation solution.
[0164] Another 250ml reaction flask was taken, and 1,3-dibromopropane (15.51g, 76.82mmol) and DMF (50ml) were added. Slowly add the indole activation solution prepared above dropwise at 0-4°C, and react at room temperature for 0.5 h after the drop is complete. After the reaction was completed, water (100ml) was added to quench the reaction, extracted with ethyl acetate, the ethyl a...
Embodiment 2
[0195] Example 2 N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(6,7,8,9-tetra Synthesis of Hydropyrido[1,2-a]indol-10-yl)-pyrimidin-2-yl)amino)phenyl)acrylamide
[0196]
[0197] Step a Synthesis of 1-(4-bromobutyl)-1H-indole
[0198]
[0199] In a 100mL reaction flask, add NaH (60% content, 1.23g, 30.73mmol) and DMF (10mL) in sequence, stir at room temperature for 5min and cool to 0-4°C, slowly add 10mL of dissolved indole (3g, 25.61mmol) DMF solution, after the addition was completed, raised to room temperature and reacted for 20 minutes to obtain an indole activation solution.
[0200] Another 250mL reaction flask was taken, and 1,4-dibromobutane (16.59g, 76.82mmol) and DMF (50mL) were added. Slowly add the indole activation solution prepared above dropwise at 0-4°C, and react at room temperature for 0.5 h after the drop is complete. After the reaction, quenched by adding water (100 mL), extracted with ethyl acetate, combined the ethyl acetate layers...
Embodiment 3
[0219] Example 3 N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((5-methyl-4-(6,7, Synthesis of 8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-yl)amino)phenyl)acrylamide
[0220]
[0221] Step a Synthesis of 10-(2-chloro-5-methylpyrimidin-4-yl)-6,7,8,9-tetrahydropyrido[1,2-a]indole
[0222]
[0223] Using the 6,7,8,9-tetrahydropyrido[1,2-a]indole and 5-methyl-2,4-dichloropyrimidine obtained in step c of Example 2 as raw materials, follow the steps in Example 2 The title compound was prepared by the method of d.
[0224] Step b N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((5-methyl-4-(6,7,8 , Synthesis of 9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-yl)amino)phenyl)acrylamide
[0225]
[0226] 10-(2-Chloro-5-methylpyrimidin-4-yl)-6,7,8,9-tetrahydropyrido[1,2-a]indole, 4-fluoro-2 -Methoxy-5-nitroaniline, N,N,N'-trimethylethylenediamine and allyl acyl chloride were used as raw materials, and the title compound was obtained according to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com