Preservation solution for peripheral blood samples of pregnant women and preservation method for peripheral blood samples of pregnant women
A technology of peripheral blood and peripheral blood of pregnant women, applied in the field of blood sample preservation, can solve the problems of inability to meet the needs of non-invasive prenatal diagnostic tests, NRBC preservation damage, etc., and achieve the effect of ensuring accuracy and delaying apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
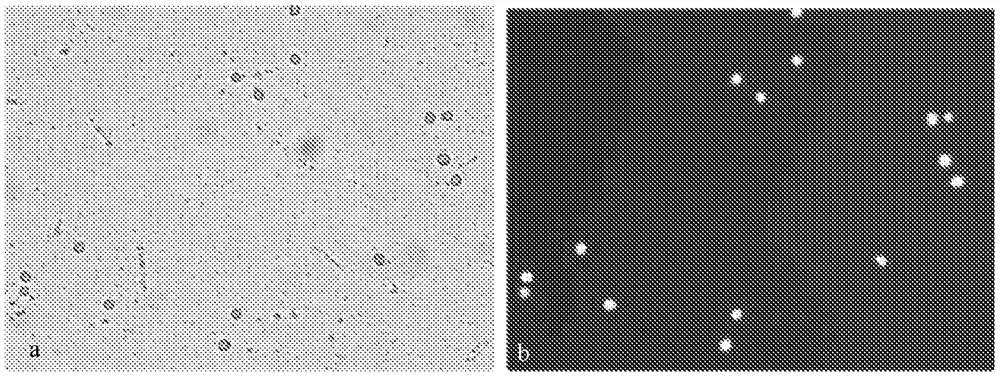
[0041] In this example, a comparative test was carried out on the components added to fresh blood samples. Take a tube of fresh pregnant women's peripheral blood for density gradient centrifugation and observe the centrifugation effect and cell status as a reference. Then set, test group a: fresh peripheral blood of pregnant women, without any addition. Experimental group b: fresh peripheral blood of pregnant women was added with Alfred's solution, and the volume ratio of pregnant women's peripheral blood to Alfred's solution was: peripheral blood of pregnant women: Alfred's solution = 1:15. Test group c: fresh peripheral blood of pregnant women, added with Caspase inhibitors, the volume ratio of peripheral blood of pregnant women to Caspase inhibitors is: peripheral blood of pregnant women: Caspase inhibitors = 1:0.1. Experimental group d: fresh peripheral blood of pregnant women was added with a preservation solution composed of Alfred's solution and Caspase inhibitor. The ...
Embodiment 2
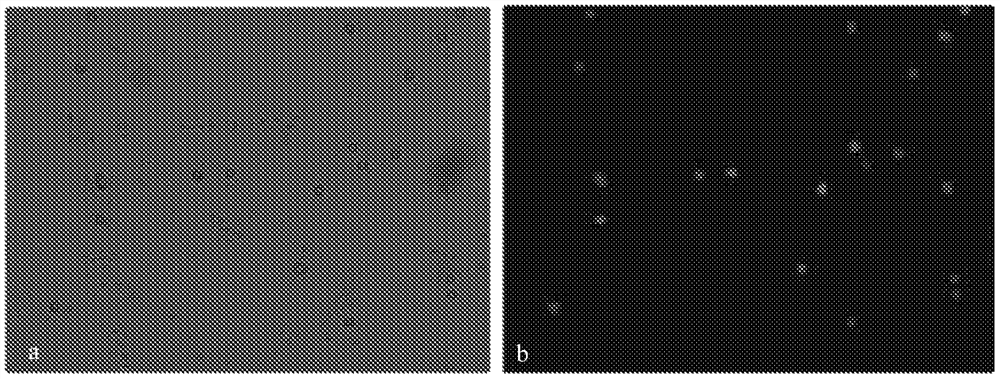
[0045] Two tubes of fresh pregnant women’s peripheral blood were drawn from the hospital, one of which was mixed with Caspase inhibitors and then transported to the laboratory at low temperature. The volume ratio of pregnant women’s peripheral blood to Caspase inhibitors was: Peripheral blood of pregnant women: Caspase inhibitors=1 -0.1. After being transported to the laboratory, the peripheral blood of the pregnant woman was added to the Alfred's solution according to the volume ratio of the pregnant woman's peripheral blood: Alfred's solution = 1:3, and stored in a refrigerator at 4°C. After 1 day, 2 days, 3 days, 5 days, and 7 days of storage, observe the morphological integrity of the cells in the blood samples and the cell viability detected by FDA+Hochest fluorescence, and perform gradient centrifugation to observe the centrifugation effect.
[0046] Another tube of pregnant women’s peripheral blood was directly stored in a 4°C refrigerator for 3 days. This sample was co...
Embodiment 3
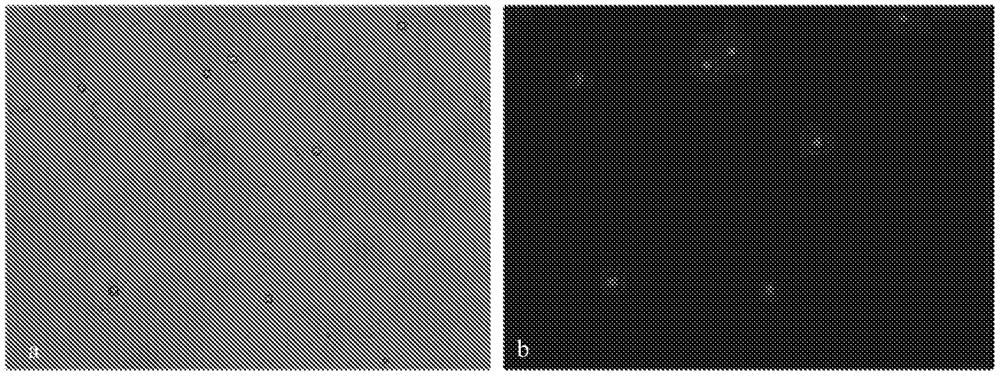
[0051] On the basis of Example 2, in this example, Alfred's solution and Caspase inhibitors were also made into a preservation solution for testing. Specifically, 1. Preservation solution prepared in the laboratory: 100ul of caspase apoptosis inhibitor was added to 15ml of Alfred's solution, the final concentration was 0.67%, mixed well, stored at -80°C in a 50ml centrifuge tube, taken out and melted before sampling room temperature. 2. Carry a 50ml preservation solution tube to the hospital at room temperature, and take peripheral blood samples from pregnant women. The pregnancy week is about 10-20 weeks. Use a 5ml heparin sodium tube, shake well and immediately transfer to a 50ml preservation solution tube. 3. The sample is placed in a low-temperature sample delivery box and quickly transported from the hospital to the laboratory, and stored in a refrigerator at 4°C. Other settings are the same as in Embodiment 2.
[0052] The results showed that the morphological integrit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com