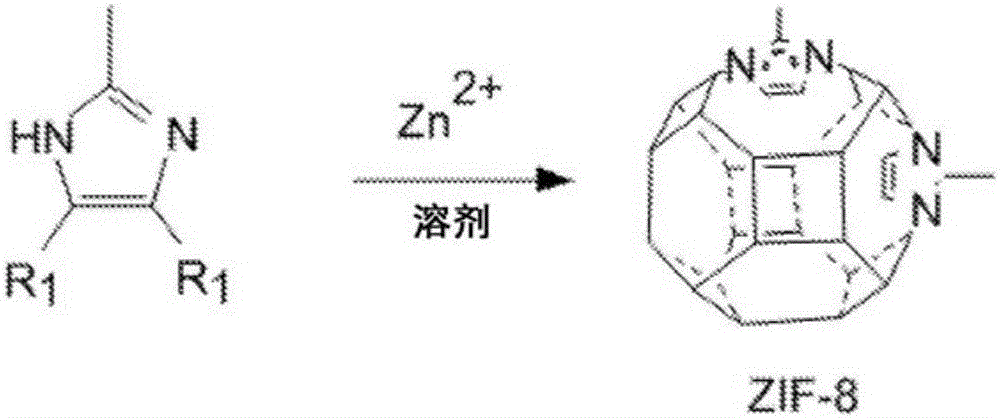
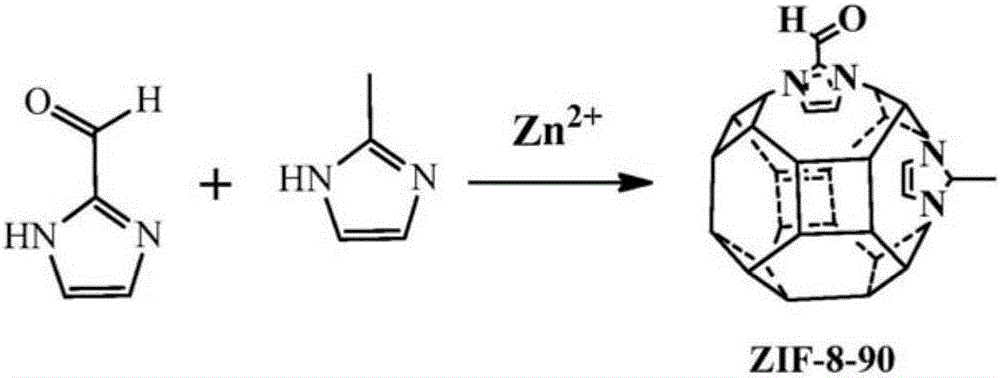
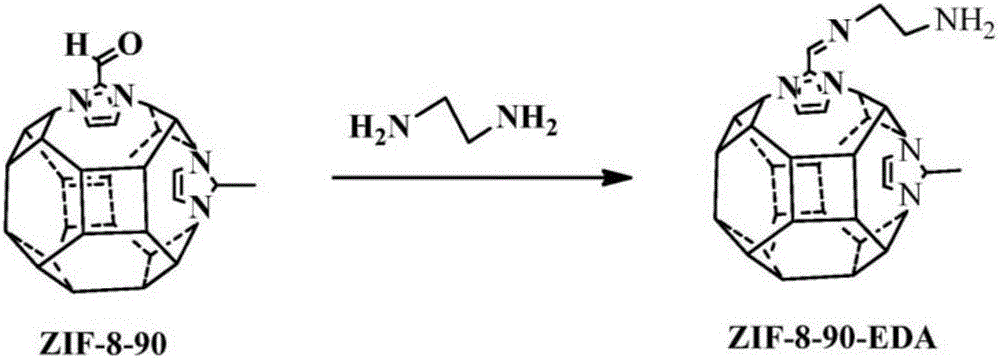
Modification of zeolitic imidazolate frameworks and azide cross-linked mixed-matrix membranes made therefrom
A technology of mixed matrix and azide compound, applied in the direction of zinc organic compound, membrane, membrane technology, etc., can solve problems such as skeleton collapse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] (Synthesis of 1,1'-oxybis(4-azidobenzene))
[0114] 4,4'-Oxydianiline (4g, 20mmol) was dissolved in water (20mL) containing concentrated HCl (11mL, 37%), cooled to 0°C, and then dissolved in sodium nitrite (3.45g, 50mmol) The solution in water (12 mL) was worked up dropwise. After the addition, the reaction was maintained at 0-5°C for 1.5 hours. To the resulting clear solution was added sodium azide (3.2 g, 5 mmol) in water (12 mL). The solution was stirred for 15 minutes. The resulting solid was collected and washed with water. A pale yellow solid was obtained by recrystallization from ethanol. Yield = 80%. The resulting solid passed 1 H-NMR (CDCl 3 ): δ7.0(s,8H) and 13 C-NMR (CDCl 3 ): δ154.3(2C), δ135.1(2C), δ120.1(8C) were characterized and confirmed to be 1,1'-oxybis(4-azidobenzene).
Embodiment 2
[0116] (Synthesis of ZIF-8 particles)
[0117] Under stirring, the Zn(NO 3 ) 2 ·6H 2 A solution of O (5 g, 16.8 mmol) in 100 mL of methanol was quickly poured into a solution of 2-methylimidazole (12 g, 146.2 mmol) in 100 mL of methanol. The mixture slowly became cloudy and after 3 hours the particles were separated from the milky dispersion by centrifugation and washed 3 times with fresh methanol. The particles were dried at 100°C under vacuum. The particle size is about 500 nm. Figure 7 is a scanning electron microscope image of ZIF-8 particles. The structure of the ZIF-8 structure was confirmed by XRD by comparing the XRD pattern with that of the simulated ZIF-8. Figure 8 are the XRD patterns of the simulated ZIF-8 (Figure 802), the XRD pattern of the synthesized ZIF-8 (Figure 804), and the XRD pattern of the ZIF-8 functionalized with the diazide of Example 1 (Figure 806). The BET surface area of the particles was determined to be about 1765.1 m 2 / g.
Embodiment 3
[0119] (Synthesis of polyimide 6FDA-DAM)
[0120] In a 250 mL three-neck round bottom flask, 4,4'-(hexafluoroisopropylidene)diphthalic anhydride (10 mmol) and 3,6-diaminodrene (10 mmol) were dissolved in anhydrous N -Methyl-2-pyrrolidone (NMP, 30mL), and in N 2 Stir under atmosphere for 24 hours. Acetic anhydride (226.6 mmol) and pyridine (11.55 mmol) were added to the reaction mixture, and the mixture was stirred for 48 hours. The resulting polymer was precipitated by pouring the solution into methanol. This precipitation process was repeated twice. A white polymer was isolated and dried under vacuum at 120°C for 48 hours. 1 H-NMR (400MHz, CDCl3): δ8.12(s, 2H), 8.00(s, 4H), 7.29(s, 1H), 2.27(s, 6H), 2.03(s, 3H). Molecular weight: Mn=3.16×10 4 g·mol -1 , PDI=2.15.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com