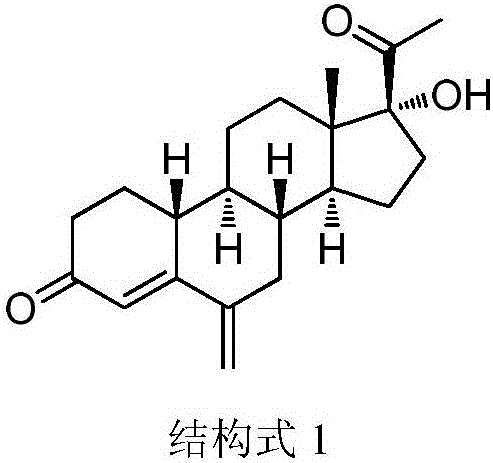
Method for synthesizing 6-methylene-17 alpha-hydroxy-19-desmethyl pregnene-4-alkene-3,20-diketone
A technology for norpregnantine and methylpregnantine, which is applied in the directions of steroids, organic chemistry and the like, can solve the problems of high cost and low yield, and achieve the effects of low cost and short synthesis process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016] The present invention will be further described below in conjunction with the examples.
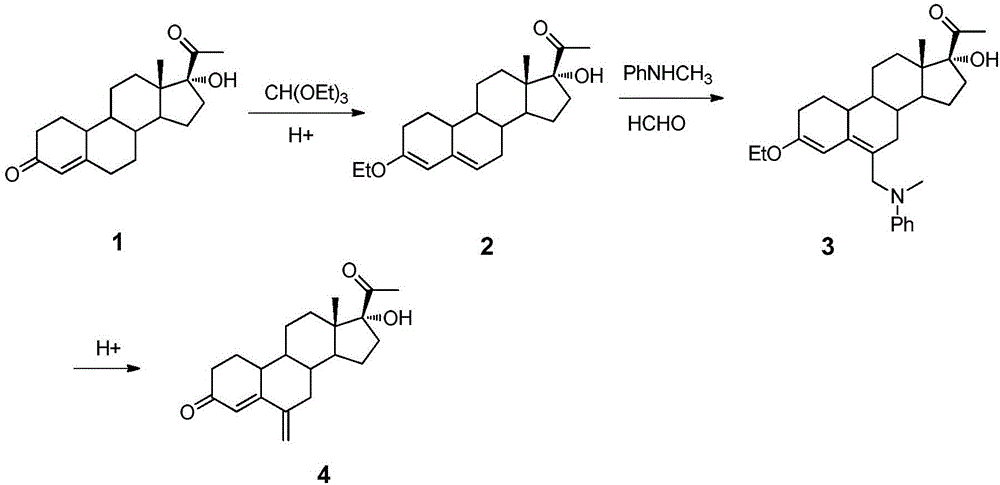
[0017] The present invention uses 17α-hydroxy-19-norpregna-4-ene-3,20-dione as raw material, and synthesizes 6-methylene-17α-hydroxy-19-norpregne 4-ene-3,20-dione, the specific route is as follows.
[0018]
[0019] 1. Preparation of 3-ethoxy-17α-hydroxy-19-desmethylpregna-4-en-20-one
[0020] 17α-Hydroxy-19-norpregna-4-ene-3,20-dione (3 g, 9.5 mmol) was dissolved in absolute ethanol (15 mL), p-toluenesulfonic acid monohydrate (0.07 g) was added, and then Triethyl orthoformate (2.82g, 19mmol) was added, the reaction solution was stirred at 20°C for 30min, heated to 80°C and continued to stir and react for 30min, most of the solvent was concentrated under reduced pressure, a small amount of ethyl acetate was added to help dissolve, and an excess of petroleum oil was added. The ether was nearly turbid, placed in a -7°C refrigerator overnight, the precipitated crystals were filte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com