Modification method and application of in-vitro immunocyte preparation
A technology of immune cells and preparations, applied in the field of cell biology, can solve problems such as failure to improve function, difficulty in obtaining NSCs, and body damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] 1. Animals and materials
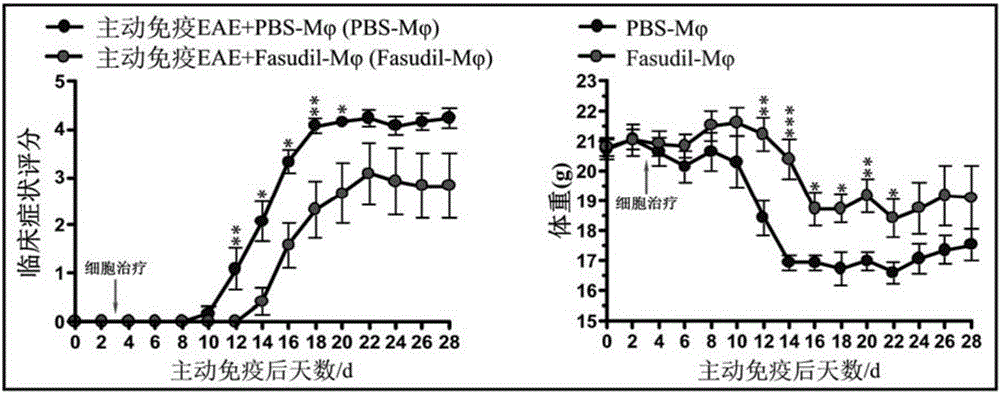
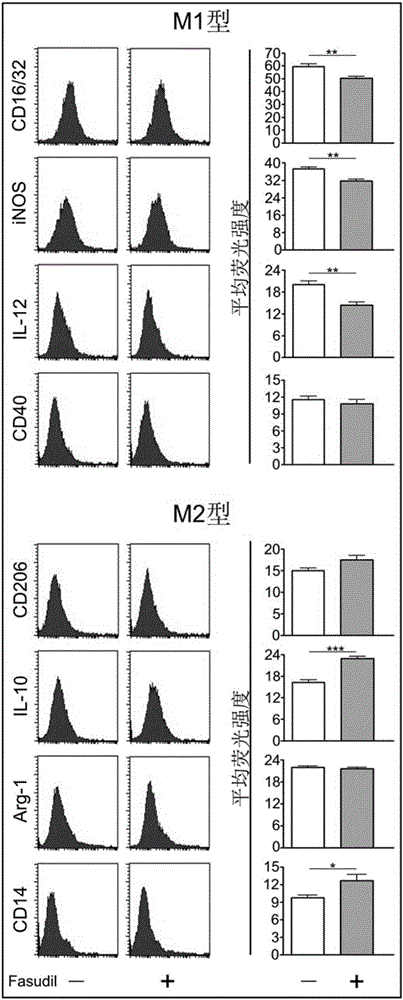
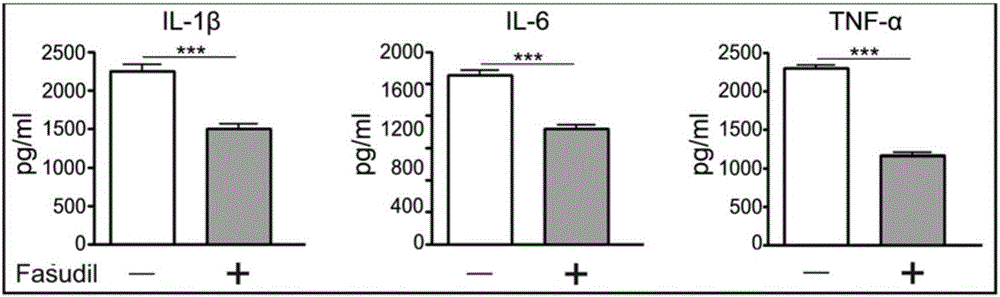
[0032] 100 female C57BL / 6 mice aged 8-10 weeks were purchased from Beijing Weitong Lihua Company. Myelin oligodendrocyte glycoprotein 35-55 polypeptide (MOG 35-55 ) was prepared by Xi'an Lianmei Biotechnology Co., Ltd.; pertussis toxin (PTX) was purchased from ALEXIS company; complete Freund's adjuvant (CFA), saponin (saponin), rabbit anti-mouse CD4 antibody was purchased from Sigma company; Mycobacterium tuberculosis (TB), PE-labeled IFN-γ, IL-10, IL-17, TGF-β, CD16 / 32, IL-12, CD40, CD206, CD14, Arginase-1 (Arg-1) antibody purchase From BD Company; rabbit anti-mouse nitric oxide synthase (iNOS) antibody was purchased from EnZo Company; fetal bovine serum (BSA) was purchased from Gibco Company; IL-10, IFN-γ, IL-17, IL-1β, TNF -α, IL-6 assay ELISA kits were purchased from Peprotech, USA; CD11b MicroBeads, T cell MicroBeads, and LS separation columns were purchased from Miltenyi, Germany; Fasudil was purchased from Tianjin Hongri Pharmaceutica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com