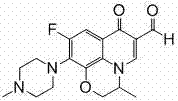
Ofloxacin aldehyde 4-arylthiosemicarbazone derivatives and preparation method and use thereof
A technology of ofloxacin aldehyde and arylamino group, which is applied in the fields of new drug discovery and innovative drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
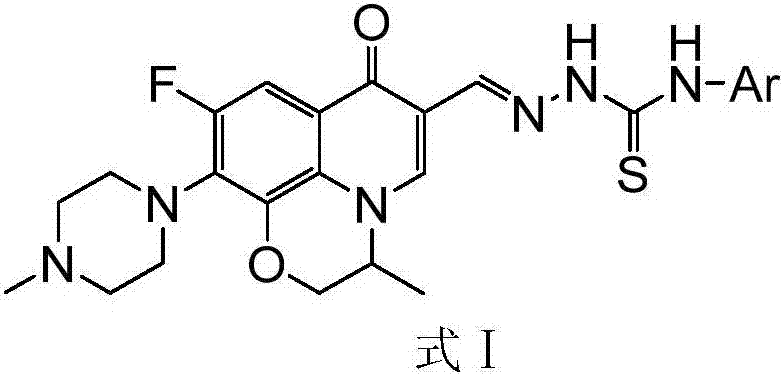
[0033] 6-Fluoro-7-(4-methyl-piperazin-1-yl)-1,8-(2,1-oxopropyl)-quinolin-4(1H)-one-3-aldehyde acetal 4- Thiosemicarbazide (I-1), its chemical structural formula is:
[0034]
[0035] That is, Ar in formula I is a benzene ring.
[0036] The preparation method of this compound is: the ofloxacin aldehyde crude product (1.0g) shown in formula IV is dissolved in absolute ethanol (30 milliliters), adds 4-phenylthiosemicarbazide (0.6g, 3.6 g) shown in formula VIII mmol), reflux reaction for 10 hours, filtered while hot, the solid was washed twice with ethanol and distilled water twice, dried, and recrystallized with a mixed solvent of DMF-ethanol (V:V=5:3) to obtain light yellow crystals According to formula (I-1), 0.72 g of product was obtained, m.p.>250°C. 1 H NMR (400MHz, DMSO-d 6 ): 11.79(s, 1H, CH=N), 9.96(s, 1H, NH), 8.91(s, 1H, 2-H), 8.45(s, 1H, NH), 7.57~7.21(m, 6H, Ph-H and 5-H),4.59~4.32(m,3H,OCH 2 CH), 3.24(t, 4H, piperazine-H), 2.43(t, 4H, piperazine-H), 2.23(s, 3...
Embodiment 2
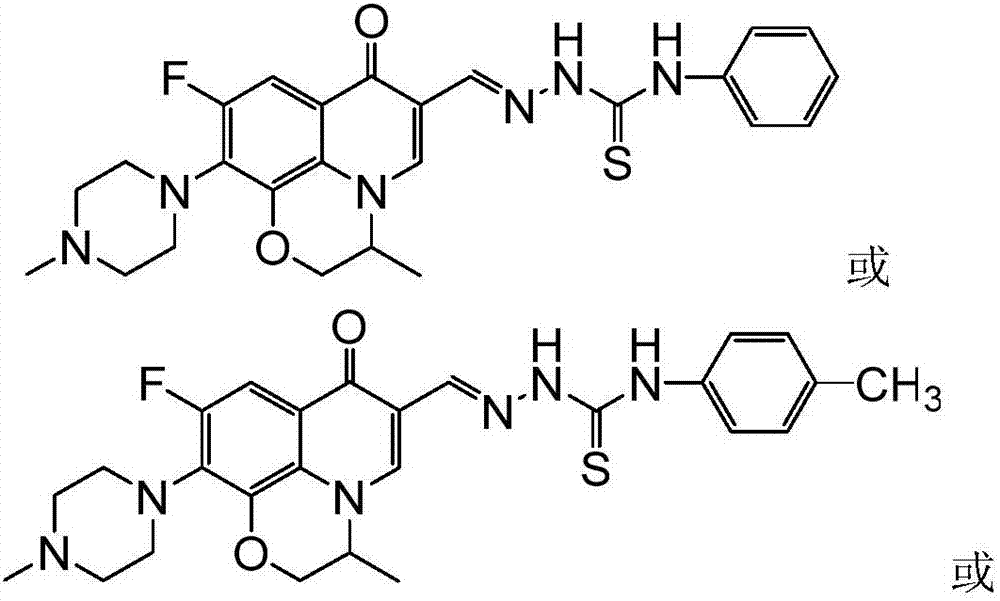
[0038] 6-Fluoro-7-(4-methyl-piperazin-1-yl)-1,8-(2,1-oxopropyl)-quinolin-4(1H)-one-3-aldehyde acetal 4- (4-methylphenyl) thiosemicarbazide (I-2), its chemical structural formula is:
[0039]
[0040] That is, Ar in formula I is 4-methylphenyl.
[0041] The preparation method of this compound is: the ofloxacin aldehyde crude product (1.0g) shown in formula IV is dissolved in absolute ethanol (30 milliliters), adds 4-(4-methylphenyl) thiosemicarbazide shown in formula VIII (0.6g, 3.3mmol), reflux reaction for 10 hours, filtered while hot, washed the solid twice with ethanol and distilled water twice, dried, and recrystallized with a mixed solvent of DMF-ethanol (V:V=5:3), The light yellow crystal formula (I-2) was obtained, and 0.67 g of the product was obtained, m.p.248-250°C. 1 H NMR (400MHz, DMSO-d 6): 11.78(s, 1H, CH=N), 9.84(s, 1H, NH), 8.92(s, 1H, 2-H), 8.46(s, 1H, NH), 8.21~7.43(m, 5H, Ph-H and 5-H),4.58~4.32(m,3H,OCH 2 CH), 3.24(t, 4H, piperazine-H), 2.44(t, 4H, ...
Embodiment 3
[0043] 6-Fluoro-7-(4-methyl-piperazin-1-yl)-1,8-(2,1-oxopropyl)-quinolin-4(1H)-one-3-aldehyde acetal 4- (4-methoxyphenyl) thiosemicarbazide (I-2), its chemical structural formula is:
[0044]
[0045] That is, Ar in formula I is 4-methoxyphenyl.
[0046] The preparation method of this compound is: the ofloxacin aldehyde crude product (1.0g) shown in formula IV is dissolved in dehydrated alcohol (30 milliliters), adds the 4-(4-methoxyphenyl) amino group shown in formula VIII Thiourea (0.7g, 3.6mmol), reflux reaction for 8 hours, filtered while hot, washed the solid twice with ethanol and distilled water twice, dried, and weighed with DMF-ethanol (V:V=5:3) mixed solvent Crystallized to obtain light yellow crystalline compound (I-3), 0.76 g of the product, m.p.>250°C. 1 H NMR (400MHz, DMSO-d 6 ): 11.73(s, 1H, CH=N), 9.86(s, 1H, NH), 8.92(s, 1H, 2-H), 8.42(s, 1H, NH), 7.45~6.94(m, 5H, Ph-H,5-H),4.58~4.31(m,3H,OCH 2 CH), 3.78(s,3H,OCH 3 ), 3.24(t, 4H, piperazine-H), 2.44(t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com