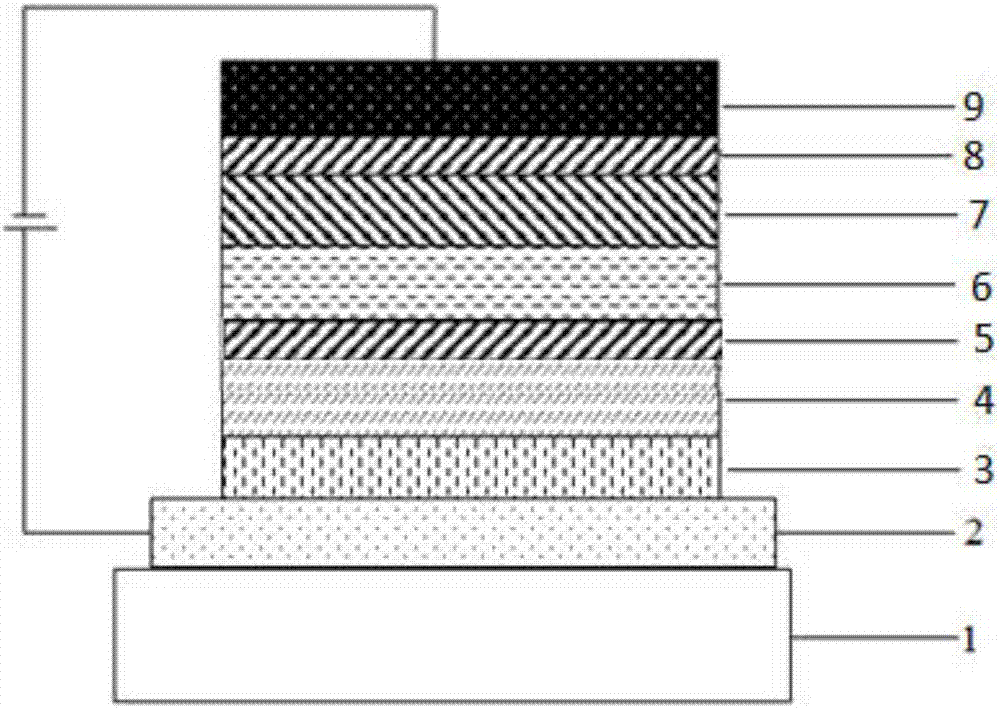
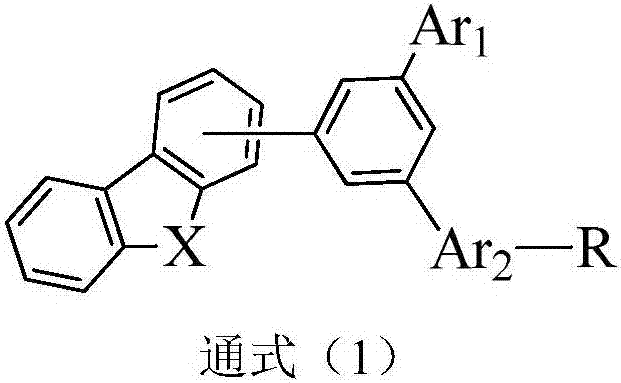
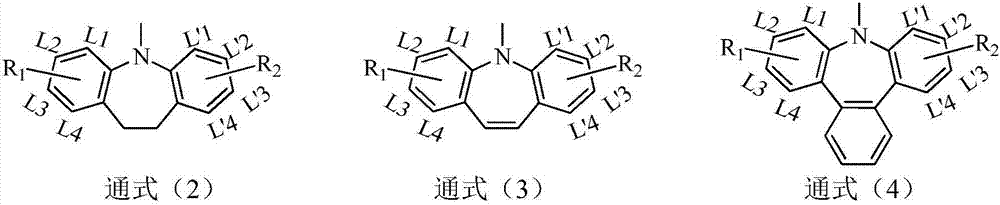
Organic compounds using homobenzenes as core and application thereof in organic electroluminescent devices
A technology of electroluminescent devices and organic compounds, applied in organic chemistry, electric solid devices, electrical components, etc., can solve problems such as performance differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1: the synthesis of intermediate I
[0069]
[0070] (1) Weigh 1,3,5-tribromobenzene and raw material U(Ar 1 -B(OH) 2 ), dissolved in a mixed solvent of toluene and ethanol with a volume ratio of 1.5 to 3.0:1; then add Na 2 CO 3 Aqueous solution, Pd(PPh 3 ) 4 ; Under an inert atmosphere, stir the above mixed solution at 95-100°C for 10-24 hours, then cool to room temperature, filter the reaction solution, spin the filtrate, and pass through a silica gel column to obtain intermediate V; the 1, The molar ratio of 3,5-tribromobenzene to raw material U is 1:1.2~2.0; Pd(PPh 3 ) 4 The molar ratio to raw material U is 0.006~0.02:1, Na 2 CO 3 The molar ratio to raw material U is 2.0-3.0:1.
[0071] (2) Weigh intermediate V and raw material W, and dissolve them in a mixed solvent of toluene and ethanol with a volume ratio of 1.5 to 3.0:1; then add Na 2 CO 3 Aqueous solution, Pd(PPh 3 ) 4 ; Under an inert atmosphere, stir the above mixed solution at 95-...
Embodiment 2
[0082] Embodiment 2: the synthesis of compound 4:
[0083]
[0084] In a 250ml three-necked flask, under a nitrogen atmosphere, add 0.01mol of intermediate A1, 0.015mol of raw material B1, 0.03mol of sodium tert-butoxide, 1×10 -4 mol Pd 2 (dba) 3 , 1×10 -4 mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampling point plate, the reaction was complete; natural cooling, filtration, filtrate rotary evaporation, silica gel column, to obtain the target product, HPLC purity 98.9%, yield 71.4%.
[0085] Elemental analysis structure (molecular formula C 50 h 35 NO): Theoretical C, 90.19; H, 5.30; N, 2.10; O, 2.40; Tested: C, 90.17; H, 5.31; N, 2.11;
[0086] ESI-MS(m / z)(M + ): The theoretical value is 665.27, and the measured value is 665.57.
Embodiment 3
[0087] Embodiment 3: the synthesis of compound 8:
[0088]
[0089] (1) In a 250ml three-necked flask, add 0.04mol raw material B1, 0.06mol 1,4-dibromobenzene, 0.12mol sodium tert-butoxide, 4×10 -4 mol Pd 2 (dba) 3 , 4×10 -4 mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampled on a plate, and the reaction was complete; naturally cooled, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain intermediate C1 with a HPLC purity of 99.3% and a yield of 62.4%.
[0090] Elemental analysis structure (molecular formula C 20 h 16 BrN): Theoretical C, 68.58; H, 4.60; Br, 22.81; N, 4.00; Found: C, 68.55; H, 4.63; Br, 22.80; N, 4.02. ESI-MS(m / z)(M + ): The theoretical value is 349.05, and the measured value is 349.37.
[0091](2) In a 250ml three-necked flask, under a nitrogen atmosphere, add 0.02mol of intermediate C1, 40ml of tetrahydrofuran to dissolve completely, cool to -78°C, and then add 15mL of 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com