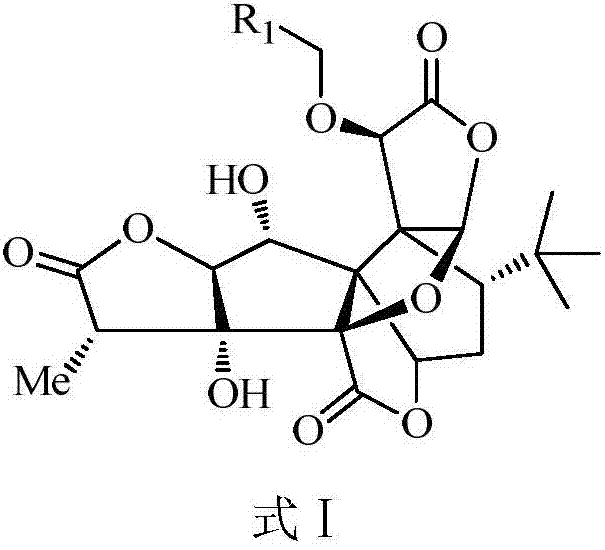
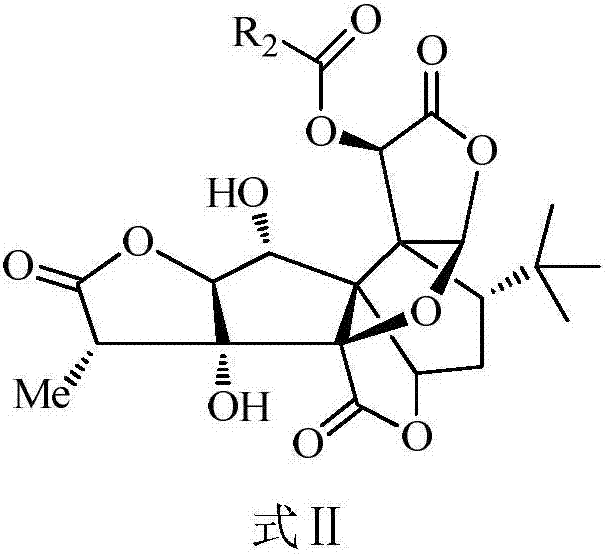
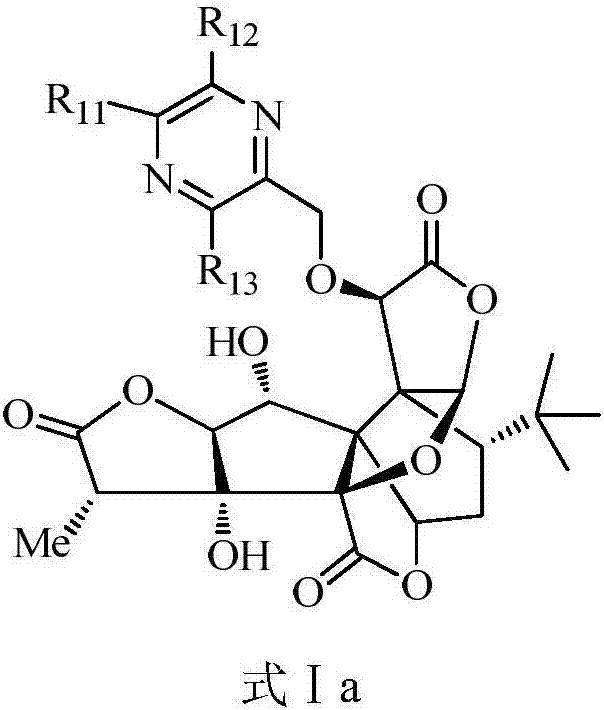
Ginkgolide B derivative as well as preparation method and application thereof
A ginkgolide and ester-based technology, which is applied to ginkgolide B derivatives and the fields of preparation and application thereof, can solve the problem of harsh and complex synthesis process, low yield, lack of test data for improvement of water solubility and pharmacodynamic activity, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1, the preparation of compound BZ
[0077]
[0078] Weigh 5.0 g (11.78 mmol) of GB and 2.55 g (15.32 mmol) of 3,5,6-trimethylpyrazine-2-carboxylic acid, dissolve them in acetonitrile, and stir and mix them in an ice bath. Then add 0.29g (2.36mmol) 4-dimethylaminopyridine (DMAP) and 3.17g (16.49mmol) 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC. HCl), after stirring for 1 hour under ice-bath conditions, the mixture was reacted at 20 ° C for 6 h, the solvent was removed by rotary evaporation, the crude product was dissolved with ethyl acetate, and 5% NaHCO 3 Wash twice, and wash once with saturated sodium chloride solution. The organic phase was collected, dried, filtered and concentrated. After separation and purification, a total of 2.80 g of white solid BZ was obtained, with a yield of 41.48% and an HPLC purity of 99.80%.
[0079] LC-MS: 573.2 [M+H + ], 595.2 [M+Na + ].
[0080] 1 H-NMR (DMSO, 400MHz): 1.02(s, 9H, t-Bu), 1.13-1.1...
Embodiment 2
[0081] Embodiment 2, the preparation of compound BA
[0082]
[0083] Weigh 5.0g (11.78mmol) GB and 2.76g (15.32mmol) acetylsalicylic acid dissolved in acetonitrile, stir and mix in an ice bath. Then add 0.29g (2.36mmol) 4-dimethylaminopyridine (DMAP) and 3.17g (16.49mmol) 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC. HCl), after stirring for 1 hour under ice-bath conditions, the mixture was reacted at 30 ° C for 4 h, the solvent was removed by rotary evaporation, the crude product was dissolved with ethyl acetate, and the mixture was washed with 5% NaHCO 3 Wash twice and once with saturated NaCl. The organic phase was collected, dried, filtered and concentrated. After separation and purification, a total of 2.52 g of white solid BA was obtained, with a yield of 36.52% and an HPLC purity of 99.13%.
[0084] MS: 609.16 [M+Na + ], C 29 h 30 NaO 13 .
[0085] 1 H-NMR (CDCl 3 , 400MHz): 1.08(s,9H,t-Bu),1.24-1.26(d,3H,14-Me)1.99-2.03(dd,1H,8-H),2.16(s,...
Embodiment 3
[0086] Embodiment 3, the preparation of compound BL
[0087]
[0088] Weigh 5.0g (11.78mmol) GB and 4.70g (15.32mmol) S(+)-2-(2-chlorophenyl)-2-(4,5,6,7-tetrahydrothiophene[3,2- c] Pyridine-5) acetic acid was dissolved in acetonitrile and stirred in an ice bath to mix. Then add 0.29g (2.36mmol) 4-dimethylaminopyridine (DMAP) and 3.17g (16.49mmol) 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC. HCl), after stirring for 1 hour under ice-bath condition, the mixture was reacted at 25° C. for 5 h. The solvent was removed by rotary evaporation, the crude product was dissolved in ethyl acetate, and washed with 5% NaHCO 3 Wash twice and once with saturated NaCl. The organic phase was collected, dried, filtered and concentrated. After separation and purification, a total of 3.46 g of white solid BL was obtained, with a yield of 41.09% and an HPLC purity of 99.58%.
[0089] LC-MS: 714.3 [M+H + ], 736.0 [M+Na + ].
[0090] 1 H-NMR (CDCl 3 , 400MHz): 1.05(s,9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com