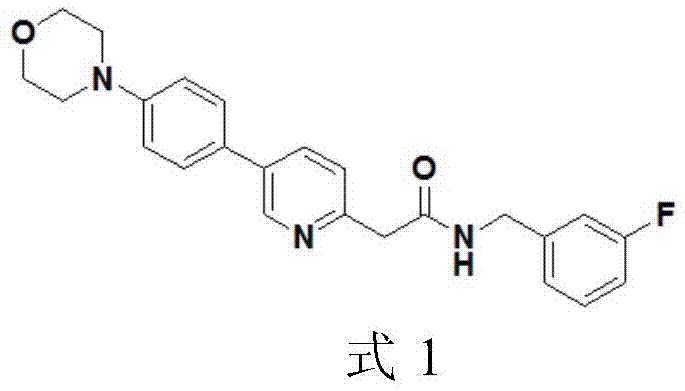
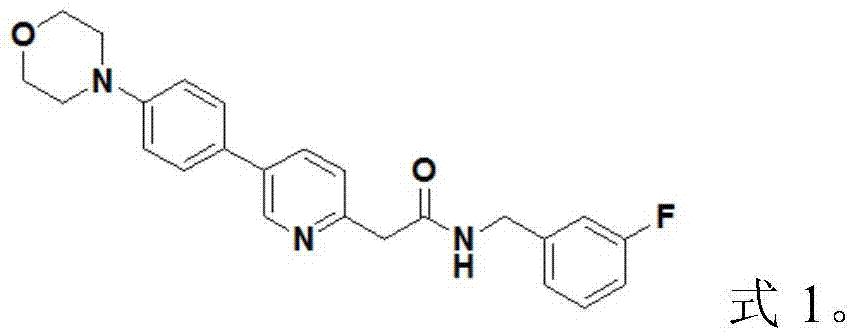
Medicine composition and application thereof, medicine inclusion compound, vein preparation and preparation method thereof
一种组合物、包合物的技术,应用在药物组合物,静脉制剂及制备领域,能够解决不适KX2-361开发成本有效、增溶方式和增溶用试剂不理想等问题,达到载药量优化、成本经济、提高溶解度的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
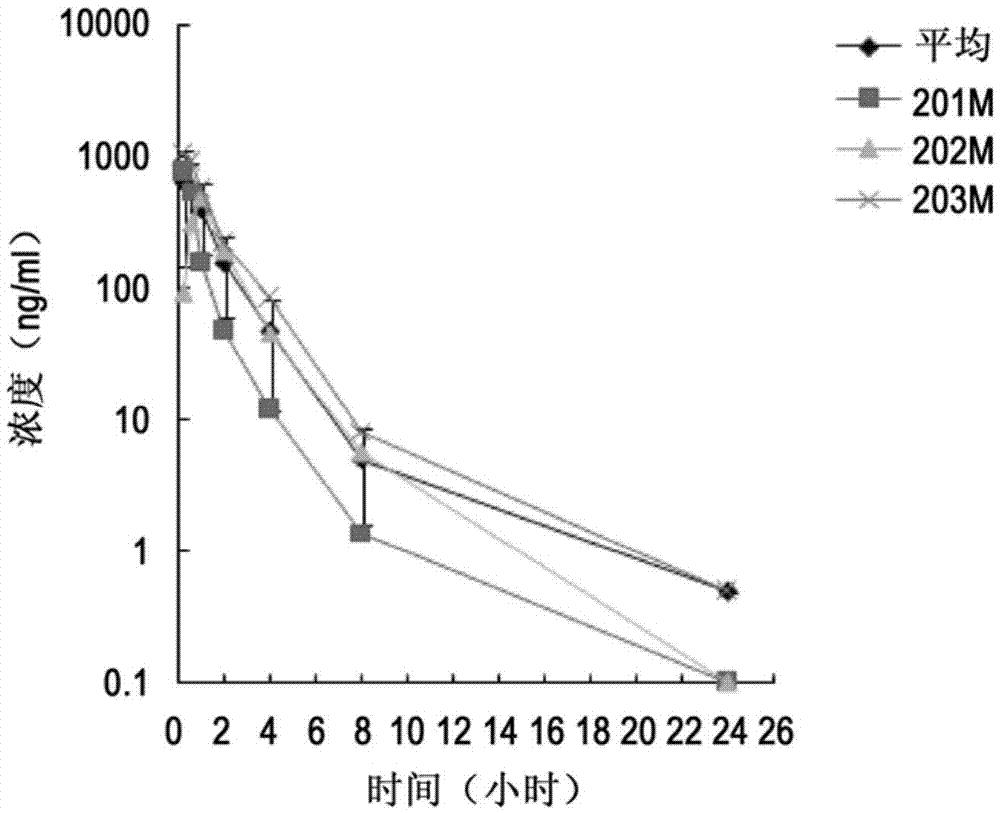
[0155] Example 1: Preparation and evaluation of KX2-361 besylate freeze-dried agent for injection and reconstitution solution (HP-β-CD concentration is 30%, high drug loading)
[0156] Prepare a prescription of the following composition:
[0157]
[0158] Note: The molecular weight of HP-β-CD is 1431~1806, so when calculating the molar ratio, the upper and lower limits of its molecular weight are used for calculation.
[0159] Follow the steps below to prepare the above prescription:
[0160] 1) Put 1.8mL of concentrated hydrochloric acid in a 200mL volumetric flask, and dilute to 200mL with distilled water to obtain a hydrochloric acid solution with a pH of about 1.2 (concentration is 0.1mol / L, the same below).
[0161] 2) Weigh HP-β-CD according to the above-mentioned prescription quantity, and add three 50mL volumetric flasks respectively. Add the solution obtained in 1), dissolve with ultrasonic (ultrasonic frequency 59kHz) and dilute to about 46mL with the above hydr...
Embodiment 2
[0192] Example 2: Preparation and evaluation of KX2-361 besylate freeze-dried agent for injection and reconstitution solution (HP-β-CD concentration is 30%, low drug loading)
[0193] Prepare a prescription of the following composition:
[0194]
[0195] Note: The molecular weight of HP-β-CD is 1431~1806, so when calculating the molar ratio, the upper and lower limits of its molecular weight are used for calculation.
[0196] Follow the steps below to prepare the above prescription:
[0197] 1) Put 1.8mL of concentrated hydrochloric acid in a 200mL volumetric flask, and dilute to 200mL with distilled water to obtain a hydrochloric acid solution with a pH of about 1.2.
[0198] 2) Weigh HP-β-CD according to the above-mentioned prescription amount, and add three 25mL volumetric flasks respectively. Add the solution obtained in 1), dissolve with ultrasonic (ultrasonic frequency 59kHz) and dilute to about 23mL with the above hydrochloric acid solution.
[0199] 3) Weigh the ...
Embodiment 3
[0260] Example 3: Preparation and evaluation of KX2-361 besylate freeze-dried agent for injection and reconstitution solution (HP-β-CD concentration is 20%, higher drug loading)
[0261] Prepare a prescription of the following composition:
[0262]
[0263] Note: The molecular weight of HP-β-CD is 1431~1806, so when calculating the molar ratio, the upper and lower limits of its molecular weight are used for calculation.
[0264] Follow the steps below to prepare the above prescription:
[0265] 1) Put 1.8mL of concentrated hydrochloric acid in a 200mL volumetric flask, and dilute to 200mL with distilled water to obtain a hydrochloric acid solution with a pH of about 1.2.
[0266] 2) Weigh HP-β-CD according to the above-mentioned prescription amount, and add to two 25mL volumetric flasks respectively. Add the solution obtained in 1), dissolve with ultrasonic (ultrasonic frequency 59kHz) and dilute to about 23mL with the above hydrochloric acid solution.
[0267] 3) APIs w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com