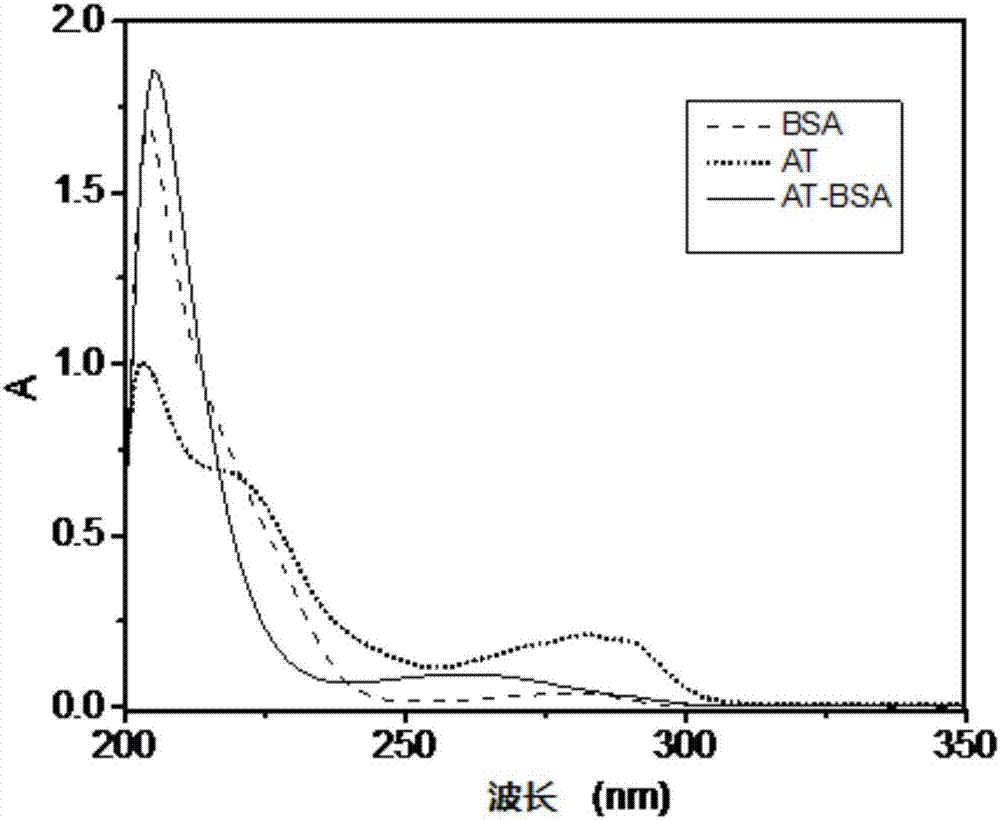
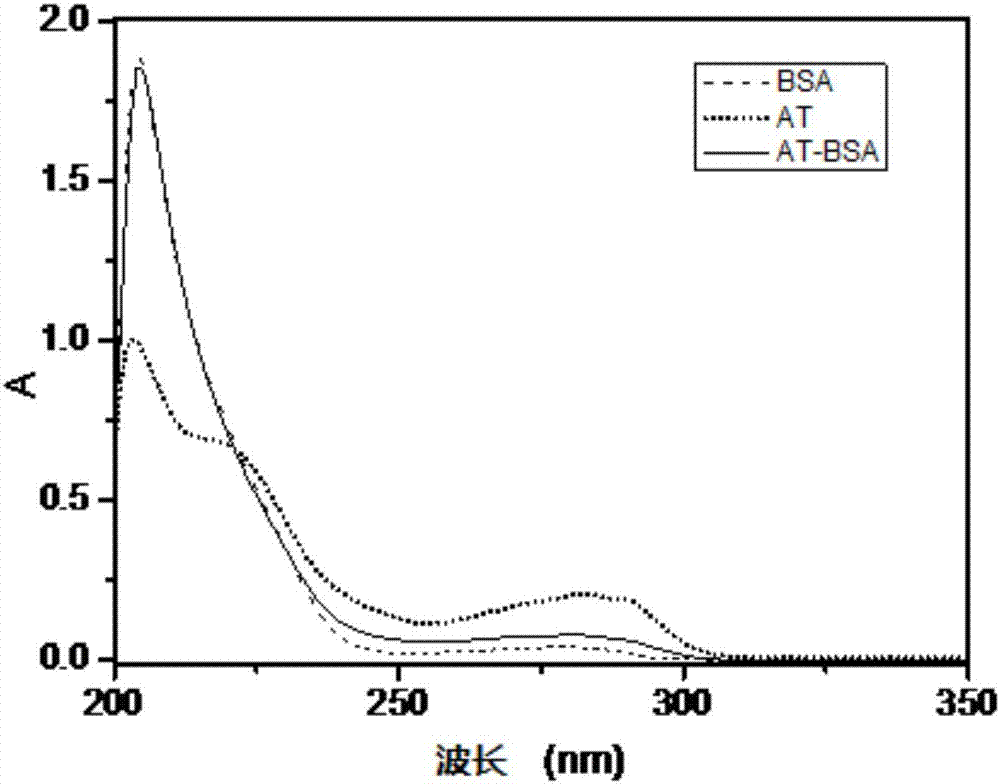
Aminotadalafil hapten, artificial antigen and preparation method thereof
An aminotadanafil and artificial antigen technology, applied in the biological field, can solve the problems of no aminotadanafil monoclonal antibody, high experimental cost, and difficulty in aminotadalafil antigens.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
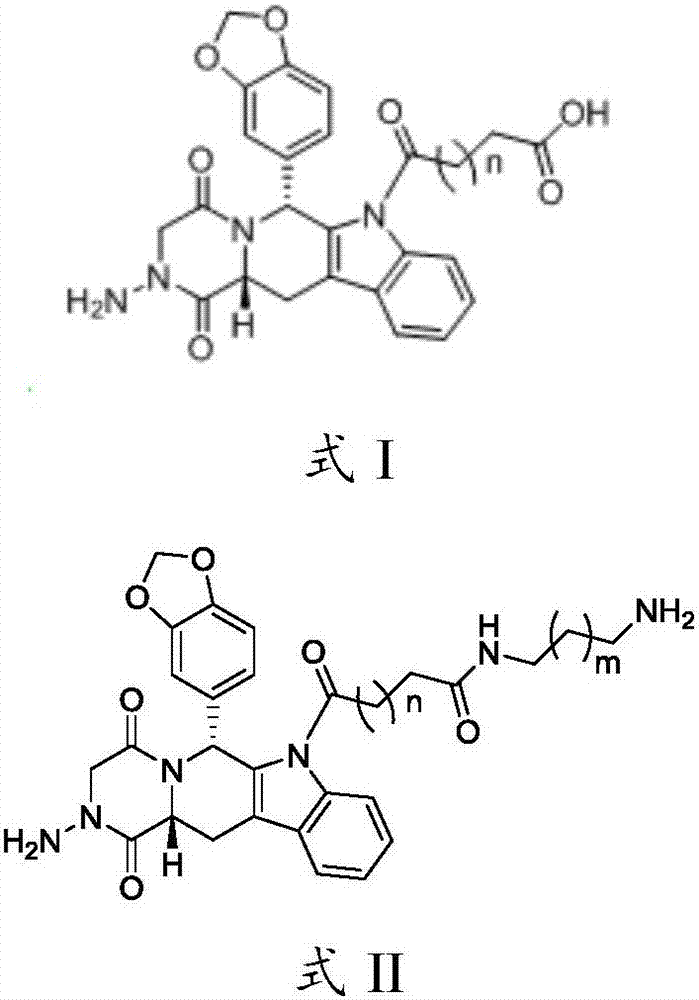
[0059] In the embodiment provided by the present invention, the preparation method of the hapten shown in formula I comprises steps:
[0060] 1) Mix 1 part of aminotadalafil with 5-13 parts of cyclic anhydride, dissolve in 30 parts of 1,4-dioxane, and then add 5 drops of triethylamine (pH 7.5) drop by drop , 40-50 ℃ airtight stirring reaction 10-12h. After the reaction, the reaction solution was poured into water, extracted 3 times with 100 parts of ethyl acetate, the combined extracts were washed with anhydrous Na 2 SO 4 After drying and concentrating, a solid is obtained, and the solid product obtained by recrystallization from 75% ethanol is an aminotadalafil derivative.
[0061] 2) Mix 1 part of aminotadalafil derivative with 4-10 parts of aliphatic diamine, stir and react at 130°C for 12 hours, and concentrate the product to near dryness under reduced pressure to obtain an oily crude product; add 50 parts of pure water, ultrasonically disperse and wash After that, the ...
Embodiment 1
[0080] Example 1 Preparation of Aminotadalafil Hapten (Scheme 1: n=1)
[0081] Mix 1 part of aminotadalafil with 10 parts of succinic anhydride, dissolve in 30 parts of 1,4-dioxane, then add 5 drops of triethylamine dropwise, and react under closed stirring at 40°C for 12 hours. After the reaction, the reaction solution was poured into water, extracted 3 times with 100 parts of ethyl acetate, the combined extracts were washed with anhydrous Na 2 SO 4 After drying and concentrating, a solid was obtained, which was recrystallized from 75% ethanol to obtain a solid product that was an aminotadalafil derivative (hapten).
[0082] The structure of the resulting product is characterized by: 1 H NMR (400MHz, DMSO) δ7.72(d, J=7.5Hz, 2H), 7.51(t, J=7.8Hz, 1H), 6.98(s, J=7.9Hz, 1H), 6.91(d, J =7.9Hz,1H),6.86(t,J=7.3Hz,1H),6.79(d,J=7.9Hz,1H),6.21(s,1H),5.94(s,2H),4.83(t,J =28.1Hz, 1H), 3.98(s, 2H), 3.07–2.95(m, 2H), 2.63(t, 2H), 2.50(t, 2H).
Embodiment 2
[0083] Example 2 Preparation of Aminotadalafil Hapten (Scheme 1: n=2)
[0084] After mixing 1 part of aminotadalafil with 10 parts of glutaric anhydride, the subsequent steps are the same as in Example 1.
[0085] The structure of the resulting product is characterized by: 1 H NMR (400MHz, DMSO) δ7.85(d, J=7.8Hz, 2H), 7.41(t, J=7.7Hz, 1H), 7.01(s, J=7.3Hz, 1H), 6.91–6.87(m ,J=8.3Hz,2H),6.80(t,J=7.3Hz,1H),6.19(s,J=8.3Hz,1H),5.94(s,J=7.6Hz,2H),4.82(t,J =8.8Hz, 1H), 4.06(s, J=4.9Hz, 2H), 3.03(d, 2H), 2.39–2.33(m, 4H), 2.10(m, J=4.2Hz, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com