NAMPT/HDAC dual-target inhibitor and preparation method thereof
A technology of derivatives and amides, which is applied in the application field of drugs for the treatment of malignant tumors and diseases related to differentiation and proliferation, and can solve problems such as dose dependence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
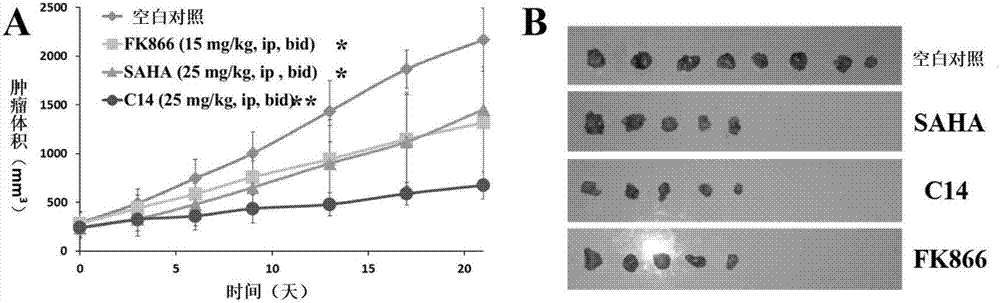
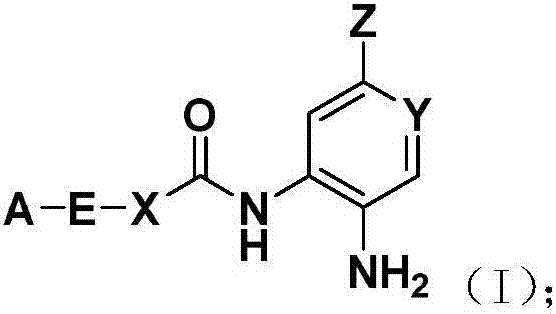
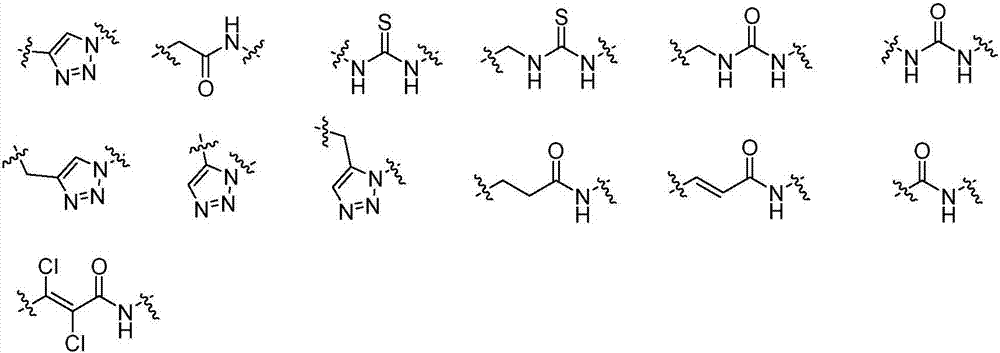
Image
Examples
Embodiment 1
[0148] Embodiment 1, N-(2-aminophenyl)-4-(3-(6-fluoropyridin-3-yl)methyl)thioureido)benzamide preparation (A1)
[0149] 1.1 Preparation of intermediate 2: 6-fluoro-3-aminomethylpyridine
[0150] Dissolve 6-fluoro-3-cyanopyridine (300mg, 2.46mmol) in 2mol / L ammonia / methanol solution (30mL), add Raney nickel (0.5g), H 2 The reaction was carried out at room temperature under atmosphere for 12 hours. After the reaction, the reaction solution was filtered with celite, rinsed with methanol (5mL×2), the filtrate was evaporated to dryness, and the residue was separated and purified by silica gel column chromatography (dichloromethane:methanol=100:4) to obtain 262 mg of a yellow oily solid, which was collected as The rate is 85%. 1 H-NMR (DMSO-d 6 , 300MHz) δ: 8.11-6.84(m, 3H), 3.86(s, 2H), 1.47(s, 2H).
[0151] 1.2 Preparation of intermediate 4: 4-isothiocyanatobenzoic acid
[0152] Triethylamine (1.4mL, 194mmol) was dissolved in dichloromethane (12mL), and thiocarbonyldiimida...
Embodiment 2
[0158] Embodiment 2, N-(4-((2-aminophenyl) carbamoyl) phenyl) imidazo [1,2-a] pyridine-7-carboxamide Prepare (B7)
[0159] 2.1 Preparation of Intermediate 8: Methyl 4-((imidazo[1,2-a]pyridine-7-carboxamide)methyl)benzoate
[0160] Dissolve imidazo[1,2-a]pyridine-6-carboxylic acid (200mg, 1.00mmol), EDC (230mg, 1.20mmol) and DMAP (146mg, 1.20mmol) in dry DMF (5mL), and stir at room temperature 0.5 hours. Additional methyl 4-aminomethylbenzoate (165 mg, 1.00 mmol) was added and stirring was continued for 2 hours. After the reaction, the reaction solution was poured into ten times the amount of water, and solids were precipitated. The solid was filtered, and the filter cake was dried and then column chromatographed to obtain 120 mg of the intermediate, with a yield of 37%. 1 H-NMR (DMSO-d 6 , 300MHz) δ: 9.21(t, J=5.7Hz, 1H), 9.16(s, 1H), 8.08(s, 1H), 7.90(d, J=8.3Hz, 2H), 7.73-7.60(m, 3H ), 7.44(d, J=8.4Hz, 2H), 4.57(d, J=5.8Hz, 2H), 3.84(s, 3H).ESI-MS(m / s): 310.51[M+1],...
Embodiment 3
[0166] Example 3, N-(2-aminophenyl)-4-((4-(pyridin-3-yl)-1H-1,2,3-triazol-1-yl)methyl)benzyl Amide Preparation (C1)
[0167] 3.1 Preparation of intermediate 15: methyl 4-azidomethylbenzoate
[0168] Methyl 4-bromomethylbenzoate (342 mg, 1.5 mmol) was dissolved in dry DMF (5 mL), and NaN3 (195 mg, 3 mmol) was added slowly with stirring at room temperature. Then, the reaction solution was placed in an oil bath at 80° C. for 8 hours. After the reaction, the reaction solution was poured into water (50 mL), extracted with ethyl acetate (50 mL×3), and dried over anhydrous magnesium sulfate. The solvent was evaporated to dryness to obtain 310 mg of the intermediate methyl 4-azidomethylbenzoate with a yield of 85%. 1 H-NMR (DMSO-d 6 , 300MHz) δ: 7.90(d, J=8.3Hz, 2H), 7.43(d, J=8.3Hz, 2H), 3.83(s, 3H), 3.77(t, J=7.0Hz, 2H), 3.20( t, J=7.0Hz, 2H).ESI-MS (m / s): 206.35[M+1].
[0169] 3.2 Preparation of Intermediate 16: Methyl 4-((4-(pyridin-3-yl)-1H-1,2,3-triazol-1-yl)methyl)benz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com