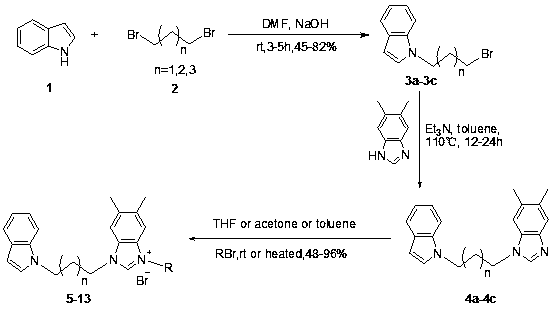
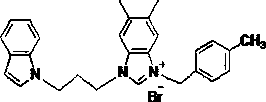
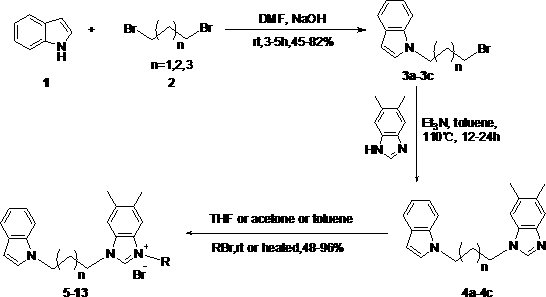
N-alkyl substituted indole-imidazolium salt compound and preparation method thereof
A technology of salt compounds and compounds, applied in organic chemistry, pharmaceutical formulations, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The preparation method specifically includes:
[0019] A, the preparation of compound N-alkyl substituted indole:
[0020] Using indole as raw material, synthesize N-alkyl substituted indole with dibromoalkane in anhydrous DMF solvent: dissolve indole in anhydrous DMF, add sodium hydroxide solid at 0 ℃, the amount is molar ratio For indole: sodium hydroxide = 1: 2, the dosage of anhydrous DMF is 20 ml: 1g indole, stir for 5 minutes, then add dibromoalkane (1,3-dibromopropane, 1,4- Dibromobutane or 1,5-dibromopentane) in anhydrous DMF, the molar ratio is dibromoalkane:indole=3:1, the amount of anhydrous DMF is 5 ml : 1 ml dibromoalkane , after stirring for 5 hours at room temperature, dilute with ethyl acetate (50 ml: 1g substrate), wash with water (50 ml) and saturated brine (50 ml) respectively, dry the organic phase with anhydrous Na2SO4, filter, and solvent After concentrating under reduced pressure, perform silica gel column chromatography with n-hexane as eluent ...
Embodiment 1
[0026] Preparation of Compound 5: See Preparation Methods A, B, and C above.
[0027]
[0028] Compound 5: Formula C 27 h 27 Br 2 N 3 , yield 69%. White solid, m.p. 108-109 o C. IR ν max (cm -1 ): 3446, 3129, 2361, 2052, 1611, 1562, 1445, 1384, 1312, 1268, 1222, 1129, 1069, 1024, 957, 860, 743, 616, 563, 428. 1 H NMR (400 MHz, DMSO): δ9.72 (1H, s), 7.75 (2H, s), 7.68 (1H, s), 7.55-7.53 (1H, d, J =7.37 Hz),7.49-7.47 (1H, d, J = 7.69 Hz), 7.41-7.35 (3H, m), 7.29-7.27 (1H, d, J = 6.73Hz), 7.12-7.09 (1H, t, J = 14.11 Hz), 7.03-7.00 (1H, t, J = 13.47 Hz), 6.43(1H, s), 5.71 (2H, s), 4.52 (2H, s), 4.36 (2H, s), 2.43 (2H, s), 2.35 (6H, s). 13 C NMR (100 MHz, DSMO): δ 141.82, 136.61, 136.54, 135.57, 133.26, 132.87,130.92, 130.37, 129.64, 129.56, 128.58, 128.47, 128.30, 128.16, 122.91,121.14, 120.55, 119.14, 113.34, 113.17, 109.87, 100.89, 50.20, 44.66, 42.80, 29.22, 20.08, 20.01.
Embodiment 2
[0030] Preparation of Compound 6: See Preparation Methods A, B, and C above.
[0031]
[0032] Compound 6: Molecular Formula C 27 h 28 BrN 3 , yield 63%. White solid, m.p. 164-165 o C. IR ν max (cm -1 ): 3448, 3128, 2320, 2051, 1644, 1561, 1456, 1354, 1262, 1128, 1069, 994,955, 861, 743, 614, 541. 1 H NMR (400 MHz, DMSO): δ 9.79 (1H, s), 9.48 (1H, s),7.71 (1H, s), 7.67 (1H, s), 7.55-7.53 (1H, d, J = 7.66 Hz), 7.48-7.46 (1H, d, J = 8.06 Hz), 7.41-7.40 (1H, d, J = 3.07 Hz), 7.39 (1H, s), 7.37 (1H, s), 7.21 (1H, s), 7.19 (1H, s), 7.13-7.09 (1H, m), 7.04-7.00 (1H, m) , 6.43-6.42(1H, d, J = 3.04 Hz), 5.59 (2H, s), 4.48-4.44 (2H, t, J = 14.76 Hz), 4.38-4.34 (2H, t, J = 13.75 Hz), 2.47-2.43 (2H, t, J= 14.29 Hz), 2.35 (3H, s), 2.34 (3H, s), 2.27 (3H, s). 13 C NMR (100 MHz, DSMO): δ 141.11, 138.04, 136.28,135.53, 131.07, 129.63, 129.44, 129.29, 128.45, 128.19, 128.13, 121.07,120.49, 119.07, 113.20, 113.08, 109.75, 100.87, 49.45, 44.51, 42.75, 29.01, 20.69, 19.99,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com