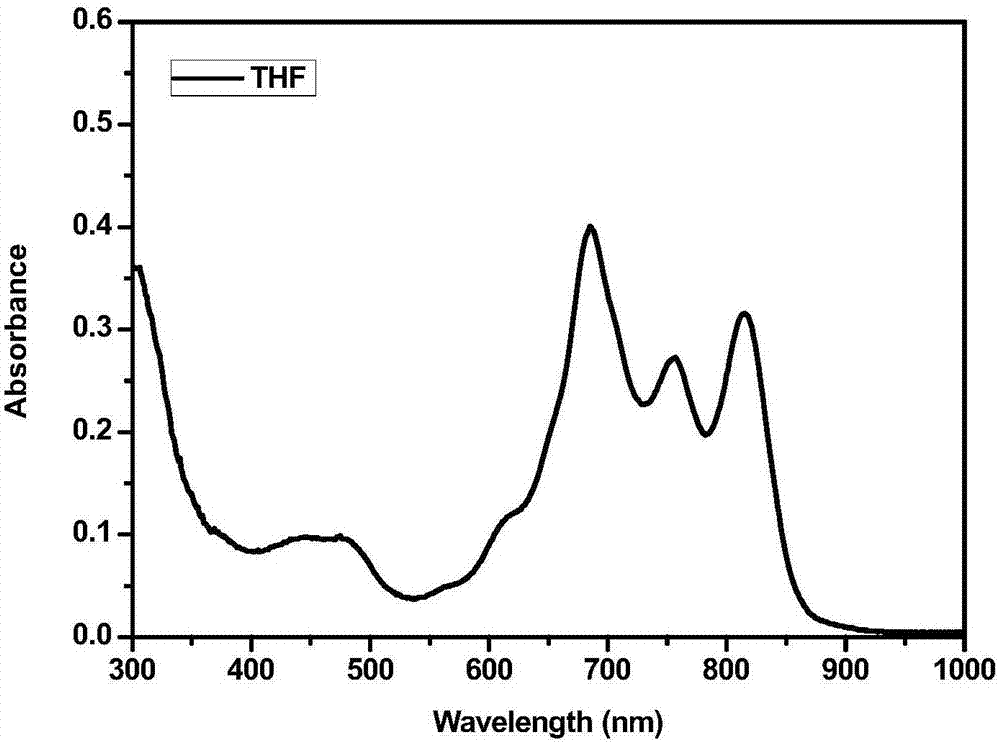
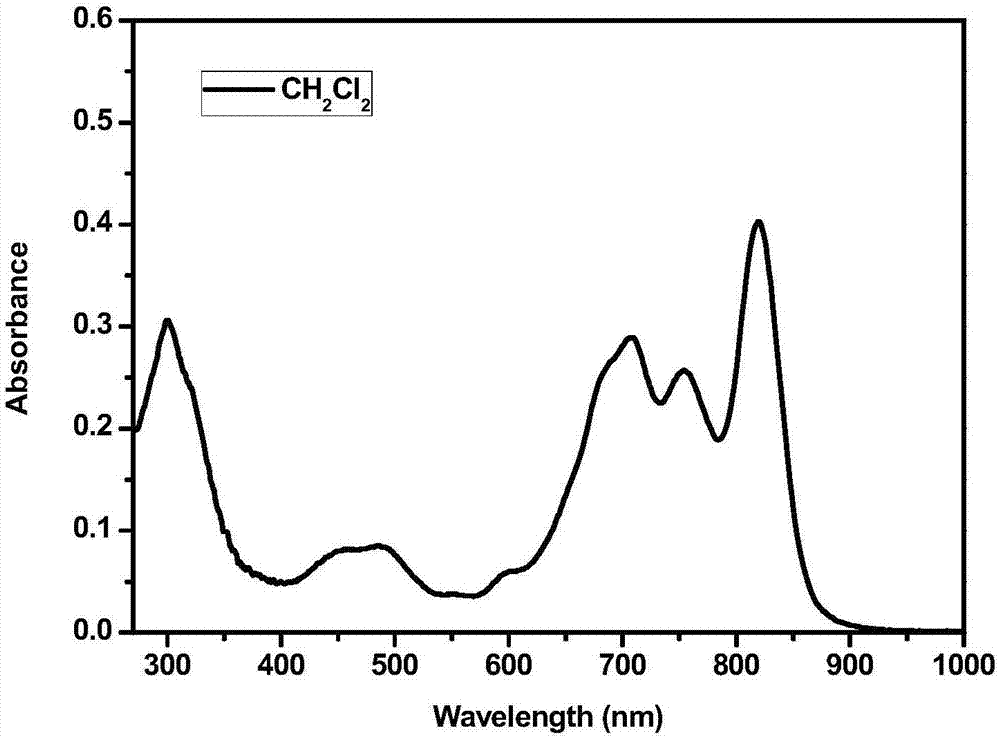
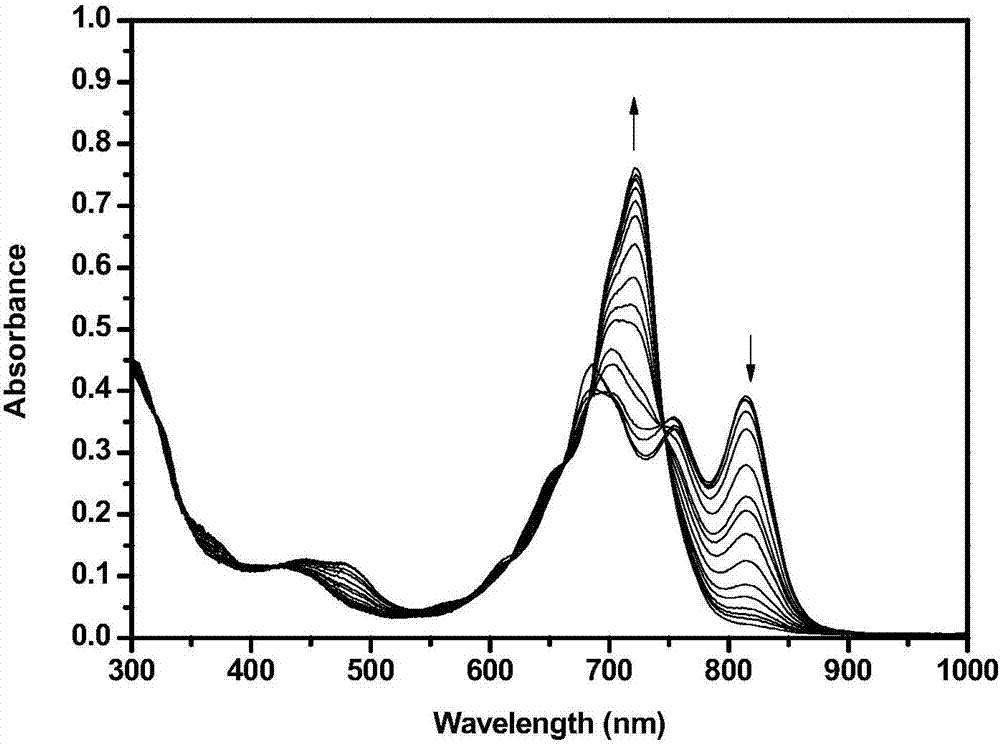
Application of phthalocyanine-iridium metal complex to detection of silver ions
A technology of iridium metal complexes and silver ions, applied in the field of chemical sensors, can solve the problems of insufficient fast detection, sample pretreatment, and expensive detection, and achieve low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] At a concentration of 1.0 x 10 -5 mol / L iridium-phthalocyanine metal complex THF solution, add 0.1 times the equivalent of AgBF 4 , so that the silver ion is 0.1 times the equivalent of the iridium-phthalocyanine metal complex, A 690 / A 814 Reduced from 1.15 to 1.13.
Embodiment 2
[0030] At a concentration of 1.0 x 10 -5 mol / L iridium-phthalocyanine metal complex THF solution, add 0.1 times the equivalent of AgBF 4 , so that the silver ion is 0.2 times the equivalent of the iridium-phthalocyanine metal complex, A 690 / A 814 Increased from 1.13 to 1.15
Embodiment 3
[0032] At a concentration of 1.0 x 10 -5 mol / L iridium-phthalocyanine metal complex THF solution, add 0.1 times the equivalent of AgBF 4 , so that the silver ion is 0.3 times the equivalent of the iridium-phthalocyanine metal complex, A 690 / A 814 Reduced from 1.15 to 1.13
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com