Preparation method of amide
A technology of amides and heterocycloalkanediones, which is applied in the preparation of lactams, carboxylic acid amides, and organic compounds, can solve the problems of reporting diazacycloalkanediones and other problems, and achieve low raw material prices , mild reaction conditions, and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
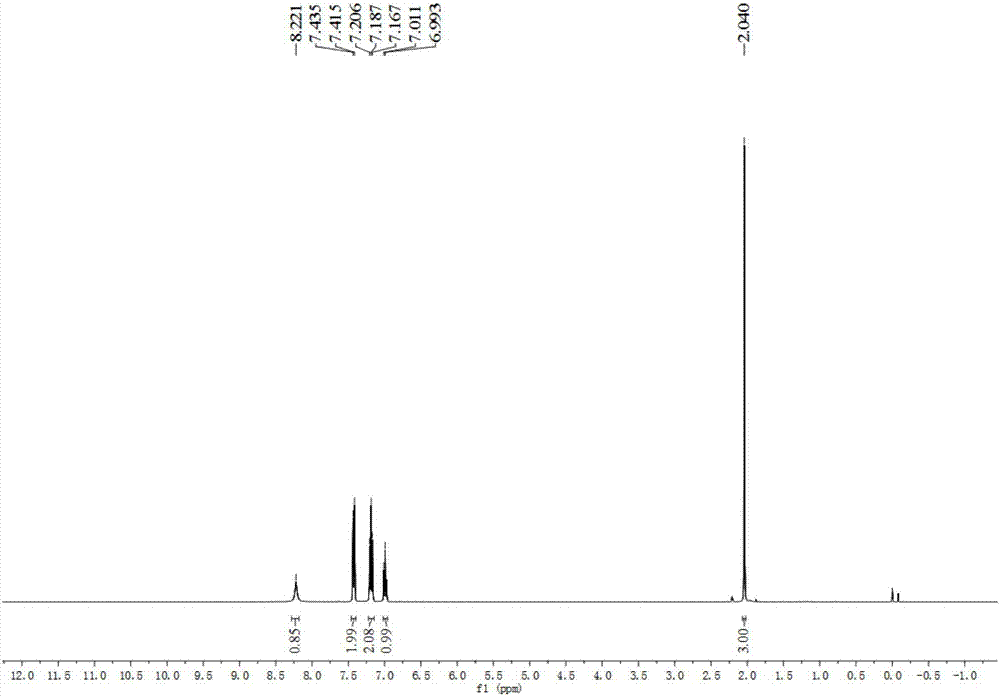
[0034] In a sealed Schlenk tube, add 2,2-dichloro-1,3-dicyclohexylimidazolidine-4,5-dione (7) (33.33 mg, 0.1 mmol), acetophenone oxime (0.135 g, 1 mmol) was dissolved in 10 mL of acetonitrile, stirred and heated to 80° C. under the protection of Ar, the raw material was completely converted in 20 min, and the selectivity of the target product acetanilide was >99%. After the reaction, the solvent was distilled off under reduced pressure, and pure acetanilide was obtained as a white solid by flash preparative chromatography with a yield of 98%. 1 H NMR (400MHz, CDCl 3 )δ8.22(s,1H),7.43(d,J=7.9Hz,2H),7.19(t,J=7.8Hz,2H),7.00(d,J=7.4Hz,1H),2.04(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ169.20, 138.14, 128.93, 124.32, 120.27, 24.41.
Embodiment 2
[0036] In a sealed Schlenk tube, add 2,2-dichloro-1,3-diisopropylimidazolidine-4,5-dione (1) (25.31 mg, 0.1 mmol), acetophenone oxime (0.135 g , 1 mmol) was dissolved in 10 mL of acetonitrile, stirred and heated to 80° C. under the protection of Ar, the raw material was completely converted in 20 min, and the selectivity of the target product acetanilide was 97%. After the reaction, the solvent was distilled off under reduced pressure, and pure acetanilide was obtained as a white solid by flash preparative chromatography with a yield of 95%. 1 H NMR (400MHz, CDCl 3 )δ8.22(s,1H),7.43(d,J=7.9Hz,2H),7.19(t,J=7.8Hz,2H),7.00(d,J=7.4Hz,1H),2.04(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ169.20, 138.14, 128.93, 124.32, 120.27, 24.41.
Embodiment 3
[0038] In a sealed Schlenk tube, add 2,2-dibromo-1,3-di-tert-butylimidazolidine-4,5-dione (2) (34.20 mg, 0.1 mmol), acetophenone oxime (0.135 g , 1 mmol) was dissolved in 10 mL of acetonitrile, stirred and heated to 80° C. under the protection of Ar, the raw material was completely converted in 20 min, and the selectivity of the target product acetanilide was 98%. After the reaction, the solvent was distilled off under reduced pressure, and pure acetanilide was obtained as a white solid by flash preparative chromatography with a yield of 96%. 1 H NMR (400MHz, CDCl 3 )δ8.22(s,1H), 7.43(d,J=7.9Hz,2H),7.19(t,J=7.8Hz,2H),7.00(d,J=7.4Hz,1H),2.04(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ169.20, 138.14, 128.93, 124.32, 120.27, 24.41.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com