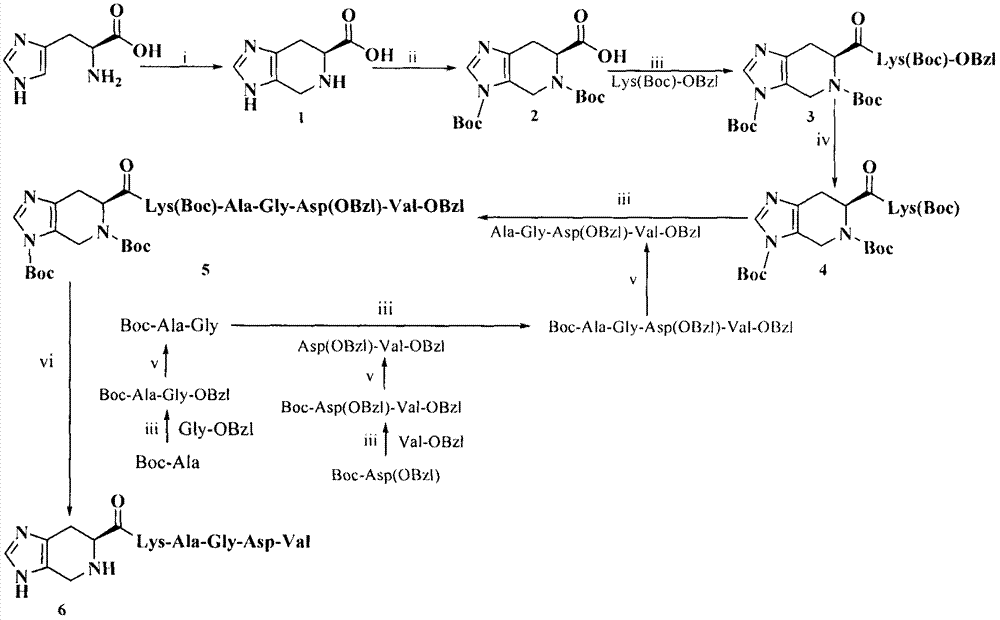
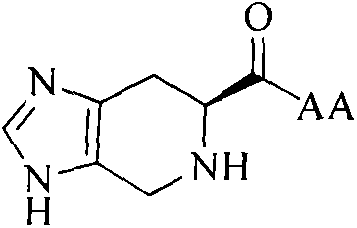
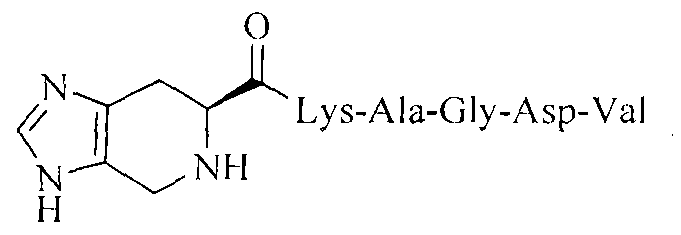Imidazopyridine acyl-KAGDV as well as synthesis, antithrombotic activity and application thereof
A technology of imidazopyridine and formyl, which is applied in the application field of antithrombotic drugs, and can solve problems such as unsatisfactory antithrombotic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Preparation of 4,5,6,7-tetrahydro-3H-imidazo[4,5-c]pyridine-6-carboxylic acid (1)
[0027] Slowly add 0.8mL H 2 SO 4 , to dissolve completely. To this solution was added 6 mL of 40% aqueous formaldehyde. The ice bath was removed, and the reaction was carried out at 60° C. for 8 h. TLC (ethyl acetate: water: glacial acetic acid = 4:1:1) monitored the disappearance of the starting point. The reaction solution was cooled to room temperature, and the pH was adjusted to 6 with concentrated ammonia water in an ice bath, and a large amount of colorless solids were precipitated. filter. The filter residue was washed with water and acetone to afford 4.52 g (84%) of the title compound. ESI-MS(m / e): 168[M+H] + .
Embodiment 2
[0028] Example 2 Preparation of 3,5-di-Boc-4,5,6,7-tetrahydro-3H-imidazo[4,5-c]pyridine-6-carboxylic acid (2)
[0029] Add 40mL of 1,4 - 14.3 g (65.7 mmol) dissolved in dioxane (Boc) 2 O, adjust the pH to 8-9 with 4N NaOH solution, react at room temperature for 24 h, and monitor the disappearance of the raw material point by TLC (dichloromethane:methanol=15:1). The reaction solution was saturated KHSO 4 Adjust the pH of the solution to 7, evaporate 1,4-dioxane under reduced pressure, and then use saturated KHSO 4 The solution was adjusted to pH 2. It was extracted three times with ethyl acetate, and the ester layer was washed three times with saturated NaCl. Dry with anhydrous sodium sulfate for 30min and filter. The filtrate was evaporated to dryness under reduced pressure. Add a small amount of ethyl acetate to just dissolve it and let it stand. A solid precipitated and was filtered. Yield 1.2 g (11%) of the title compound as a colorless solid. ESI-MS(m / e): 368[M+H]...
Embodiment 3
[0030] Example 3 Preparation of 3,5-di-Boc-4,5,6,7-tetrahydro-3H-imidazo[4,5-c]pyridine-6-formyl-Lys(Boc)-OBzl(3)
[0031] 367 mg (1 mmol) of 3,5-di-Boc-4,5,6,7-tetrahydro-3H-imidazo[4,5-c]pyridine-6 was dissolved in 20 mL of dry tetrahydrofuran (THF) under ice bath - Carboxylic acid dissolves. Add 142mg (1.05mmol) HOBt and 258mg (1.25mmol) DCC, stir and activate for 30min. Dissolve 558 mg (1.1 mmol) Tos·Lys(Boc)-OBzl in 10 mL of dry THF, adjust the pH to 8 with NMM, add the solution dropwise to the reaction solution, and finally adjust the pH of the reaction solution to 8 with NMM. After reacting at room temperature for 5 h, TLC (petroleum ether: acetone = 3:1) showed that the starting material disappeared. Dicyclohexylurea (DCU) was removed by filtration, the reaction solution was evaporated to dryness under reduced pressure, and the residue was dissolved in ethyl acetate, followed by saturated NaHCO 3 solution, saturated NaCl solution, 5% KHSO 4 solution, saturated Na...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com