A kind of preparation method of indoxacarb insecticide
A technology of indoxacarb and insecticides, which is applied in the field of preparation of indoxacarb insecticides, can solve the problems of affecting the content of indoxacarb products, difficult recovery of mixed solvents, and easy generation of impurities, so as to avoid decomposition and impurities The production and operation are simple and feasible, and the production cost is reduced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
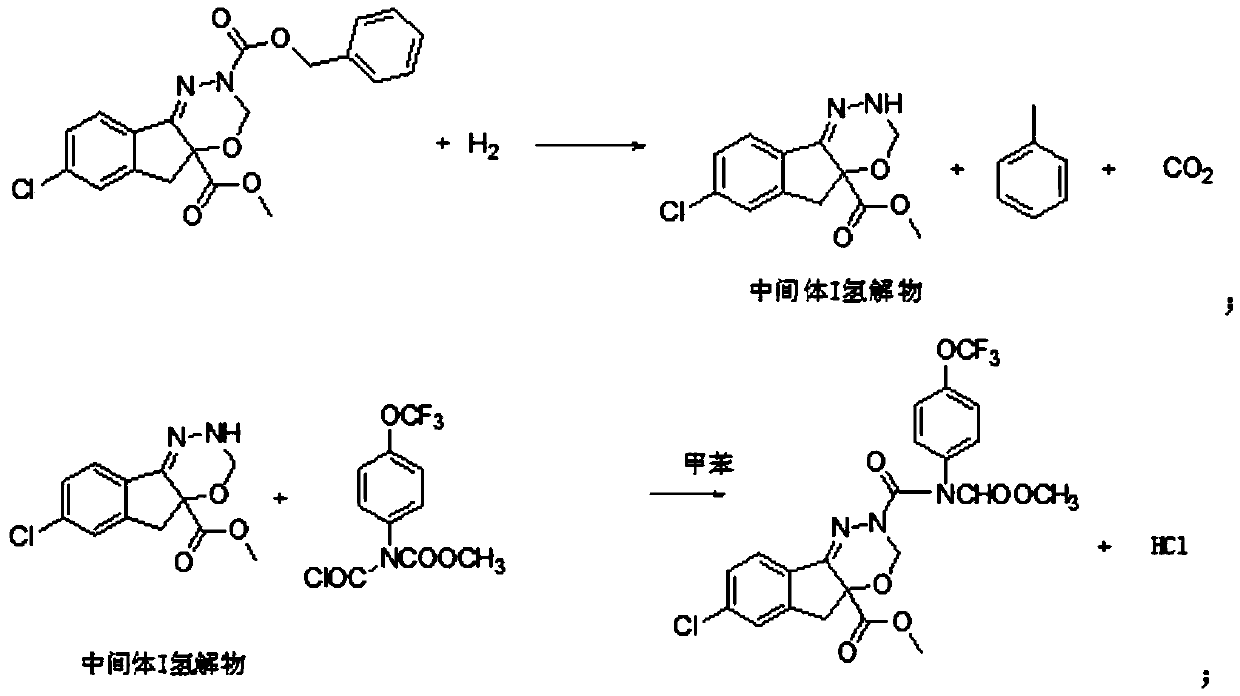
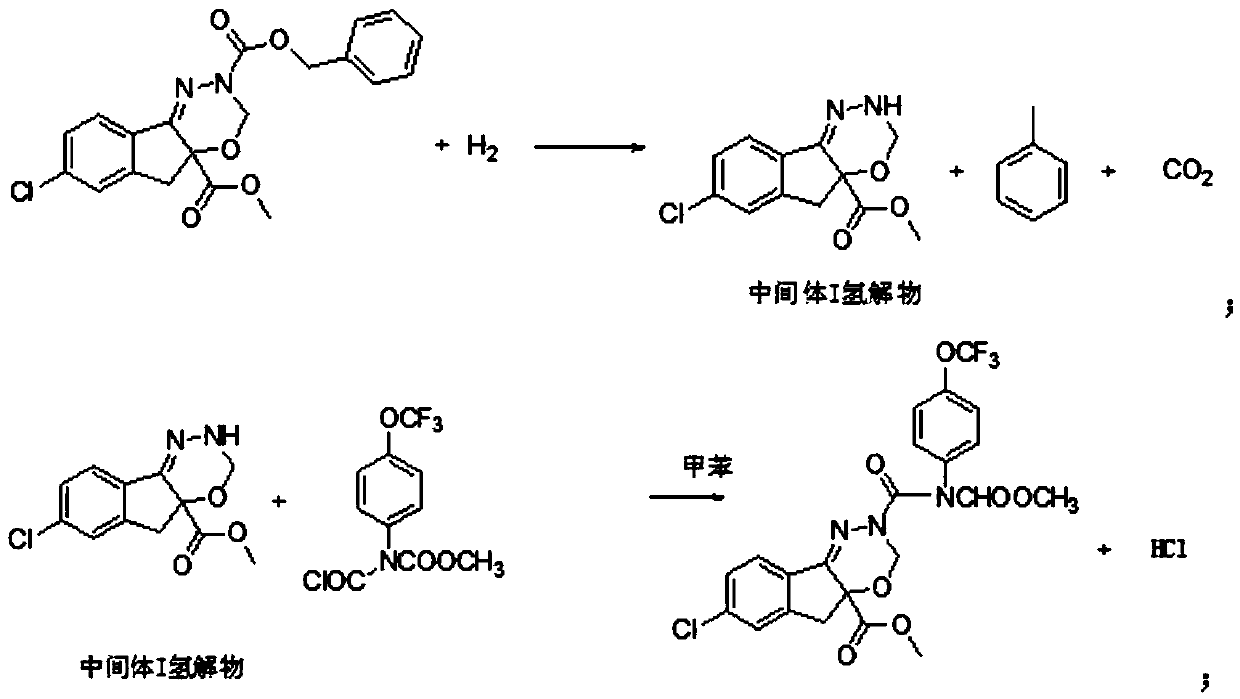
[0025] (1) 81.6g (0.2mol) 7-chloroindeno[1,2-e][1,3,4]oxadiazine-2,4a(3H,5H)- Dicarboxylic acid-4a-methyl-2-benzyl ester, 400g toluene, heat up to 25°C, then put 0.4g of Pd-C catalyst and 0.2g of tetrabutylammonium bromide into the reaction flask, start to pass hydrogen to control the hydrogen flow rate 500mL / min;
[0026] (2) Sampling and testing after hydrogenation for 4 hours, after the reaction is qualified (reaction is complete if the remaining amount of raw material is less than 1%), suction filtration; hydrogenation catalyst and phase transfer catalyst are suction filtered out (catalyst is a solid suspension, requiring suction) The filtrate is clear and transparent, and leakage cannot occur), so as to obtain the reaction solution a, the product contained in the reaction solution is the hydrogenated product of intermediate I, that is, the hydrogenation reduction product of the raw material;
[0027] (3) 74.25g N-chloroformyl-N-[4-(trifluoromethoxy)phenyl]methyl carbamat...
Embodiment 2
[0031] (1) 81.6g (0.2mol) 7-chloroindeno[1,2-e][1,3,4]oxadiazine-2,4a(3H,5H)- Dicarboxylic acid-4a-methyl-2-benzyl ester, 400g toluene, heat up to 25°C, then put 0.4g of Pt-C catalyst and 0.2g of tetrabutylammonium chloride into the reaction bottle, start to pass hydrogen to control the hydrogen flow rate 300mL / min.
[0032] (2) Sampling and testing after hydrogenation for 4 hours, after the reaction is qualified (reaction is complete if the remaining amount of raw material is less than 1%), suction filtration; hydrogenation catalyst and phase transfer catalyst are suction filtered out (catalyst is a solid suspension, requiring suction) The filtrate is clear and transparent, and there can be no leakage), to obtain the reaction solution a, and the product contained in the reaction solution is intermediate I hydrogenolyzate, that is, the hydrogenation reduction product of the raw material.
[0033] (3) 59.4g N-chloroformyl-N-[4-(trifluoromethoxy)phenyl]methyl carbamate (0.2mol)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com