Phenyl-bridged compound with triarylamine and ferrocene end groups, method for preparing phenyl-bridged compound and application thereof
A technology of monobrominated phenylferrocene and phenyl bridges, applied in the direction of iron organic compounds, etc., can solve problems such as unseen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
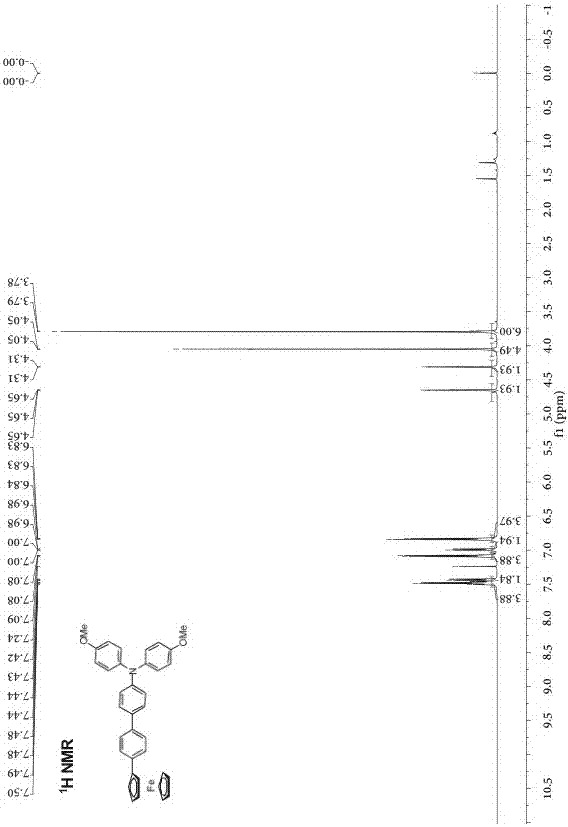
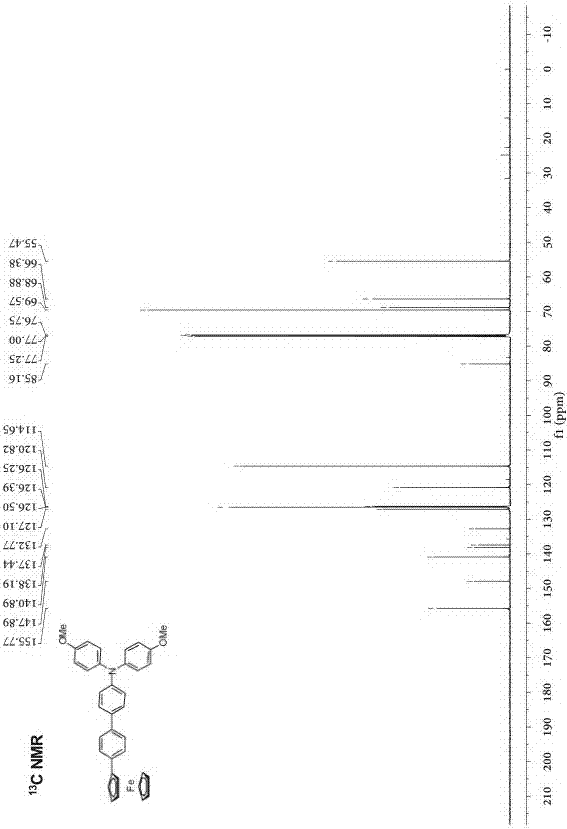
[0030] Preparation of phenyl-bridged triarylamine and ferrocene end-group compounds: add 0.2 g (0.464 mmol) of borate-substituted triarylamine and 0.143 g (0.420 mmol) of monobromophenylferrocene to a round-bottomed flask , 2 mL of potassium carbonate solution with a concentration of 2 mol / L, 2 mL of ethanol and 10 mL of toluene, the mixed system was stirred and heated to reflux for 24 h, cooled, and the mixed system was mixed with CH 2 Cl 2 solvent extraction, the obtained organic phase was washed with saturated NaCl solution, and then washed with anhydrous NaCl 2 SO 4 Dry, filter, spin the filtrate with a rotary evaporator at a pressure of 0.01Kpa and a temperature of 30°C, and separate by column chromatography (eluent: ethyl acetate / petroleum ether (V / V= 1:8) to obtain Orange-red solid 182 mg, yield: 77%.
[0031] .
[0032] Elemental analysis (C 36 h 31 FeNO 2 ): theoretical value: C, 76.46; H, 5.53. Found: C, 76.55; H, 5.38.
[0033] Structural data: 1 H NMR (...
Embodiment 2
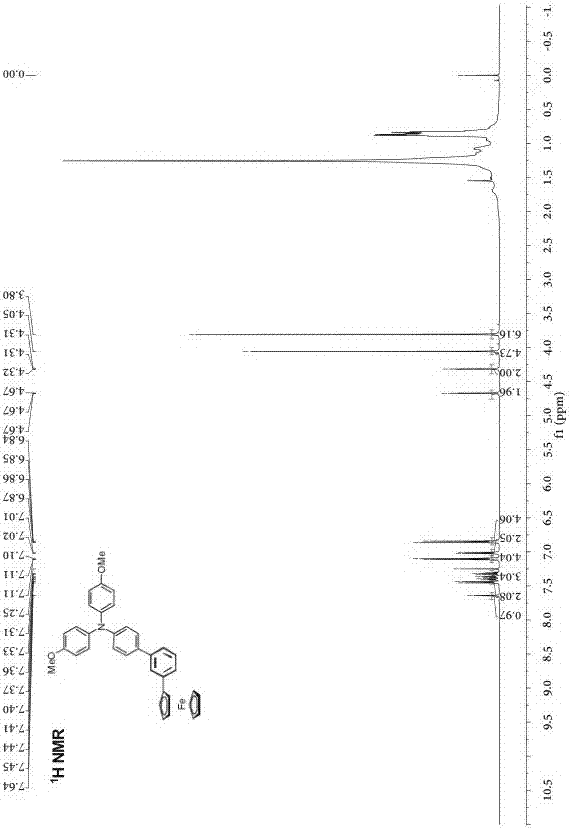
[0036] Preparation of phenyl-bridged triarylamine and ferrocene end-group compounds: add 0.2 g (0.464 mmol) of borate-substituted triarylamine and 0.143 g (0.420 mmol) of monobromophenylferrocene to a round-bottomed flask , 2 mL of potassium carbonate solution with a concentration of 2 mol / L, 2 mL of ethanol and 10 mL of toluene, the mixed system was stirred and heated to reflux for 24 h, cooled, and the mixed system was mixed with CH 2 Cl 2 solvent extraction, the obtained organic phase was washed with saturated NaCl solution, and then washed with anhydrous NaCl 2 SO 4 Dry, filter, spin the filtrate with a rotary evaporator at a pressure of 0.01Kpa and a temperature of 30°C, and separate by column chromatography (eluent: ethyl acetate / petroleum ether (V / V= 1:8) to obtain Orange-red solid 160 mg, yield: 67%.
[0037]
[0038] Elemental analysis (C 36 h 31 FeNO 2 ): theoretical value: C, 76.46; H, 5.53. Found: C, 76.41; H, 5.60.
[0039] Structural data: 1 H NMR (50...
Embodiment 3
[0042] Preparation of phenyl-bridged triarylamine and ferrocene end-group compounds: add 0.4 g (0.928 mmol) of borate-substituted triarylamine and 0.286 g (0.840 mmol) of monobromophenylferrocene to a round-bottomed flask , 5 mL of potassium carbonate solution with a concentration of 2 mol / L, 5 mL of ethanol and 20 mL of toluene, the mixed system was stirred and heated to reflux for 24 h, cooled, and the mixed system was mixed with CH 2 Cl 2 solvent extraction, the obtained organic phase was washed with saturated NaCl solution, and then washed with anhydrous NaCl 2 SO 4 Dry, filter, spin the filtrate with a rotary evaporator at a pressure of 0.01Kpa and a temperature of 30°C, and separate by column chromatography (eluent: ethyl acetate / petroleum ether (V / V= 1:8) to obtain Orange-red solid 266 mg, yield: 56%.
[0043] .
[0044] Elemental analysis (C 36 h 31 FeNO 2 ): theoretical value: C, 76.46; H, 5.53. Found: C, 76.39; H, 5.62.
[0045] Structural data: 1 H NMR (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com