A kind of large sterically hindered alkoxyl substituted conjugated compound with triarylamine as terminal group and its application
A technology of conjugated compounds and hindered alkoxy groups, which is applied in the field of large sterically hindered alkoxy groups substituted conjugated compounds, can solve the problems of weak electronic mutual ability, the performance of insulating molecular wires is difficult to meet the application standards, and achieves good charge transport ability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
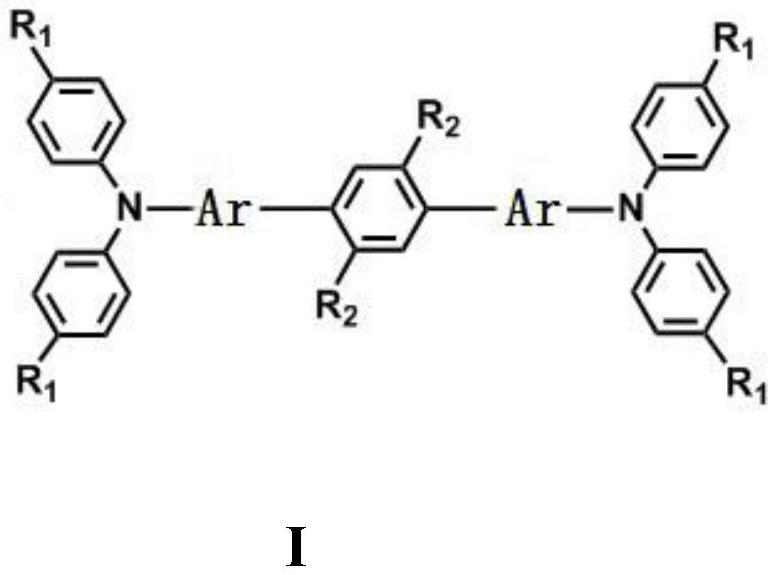
[0032] A large sterically hindered alkoxyl substituted conjugated compound with a triarylamine as an end group, the compound is bridged by a phenyl group entwined with a dendritic alkoxyl group, and its preparation method is as follows:
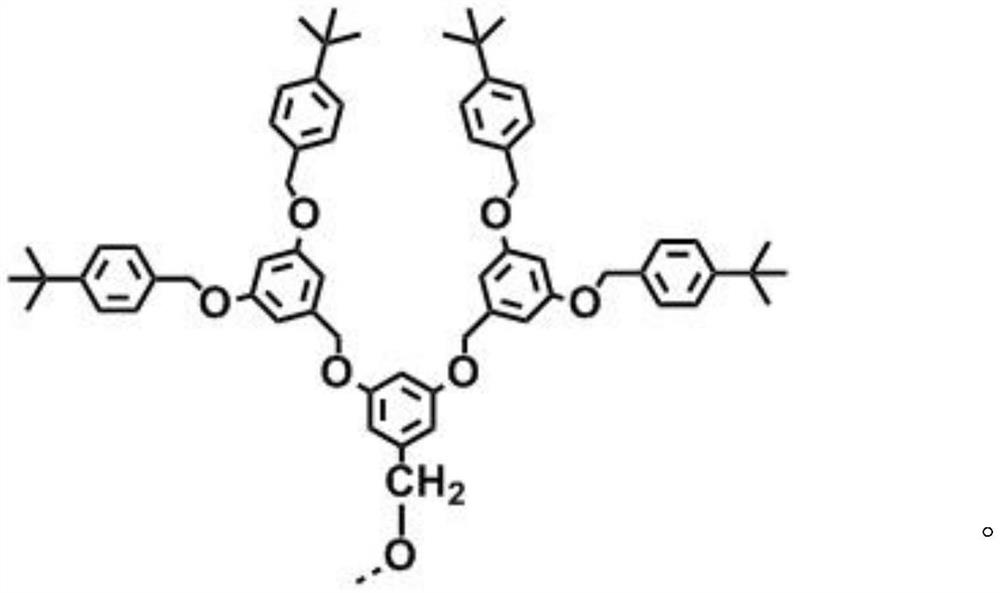
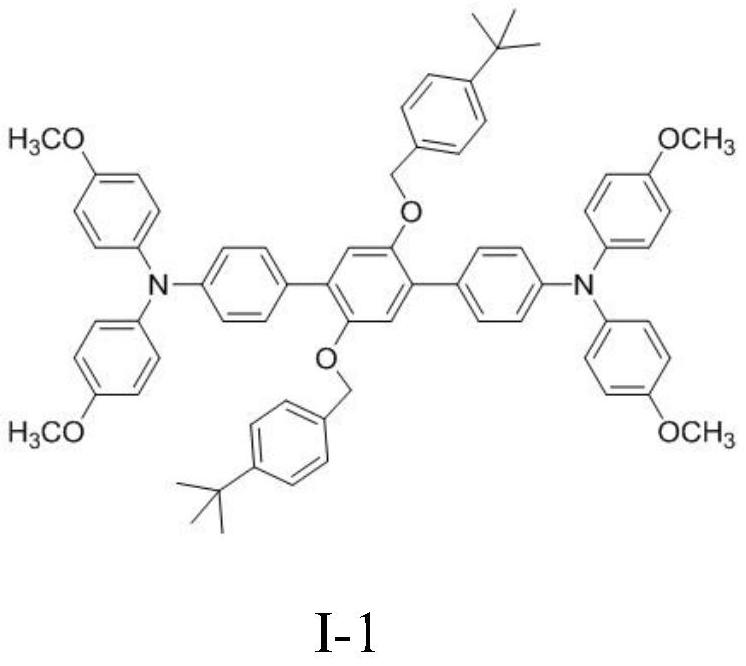
[0033] Add 0.46mmol 4-boronate-4',4'-dimethoxytriphenylamine (200mg), 0.21mmol 2,5-bis(4-tert-butylbenzyloxy)-1 to a 50mL two-necked flask , 4-diiodobenzene (137mg), 0.01mmol tetrakis (triphenylphosphine) palladium (12mg), the system was filled with N 2 Three times, and then add degassed 10mL toluene, 2mL ethanol and 2mL 2mol / L potassium carbonate solution under the protection of nitrogen, and then reflux at 110°C for 24h, the system is cooled to room temperature, filtered through diatomaceous earth to obtain the filtrate, spin Dry and separate by column chromatography to obtain 112 mg of a yellow solid with structural formula I-1, yield: 53%.
[0034] During the separation process of column chromatography, the silica gel used is 200-300 mes...
Embodiment 2
[0041] A large sterically hindered alkoxyl substituted conjugated compound with a triarylamine as an end group, the compound is bridged by a phenyl group entwined with a dendritic alkoxyl group, and its preparation method is as follows:
[0042] Add 1.71mmol 4-boronate-4', 4'-dimethoxytriphenylamine (737mg) and 0.81mmol 1,4-diiodobenzene (965mg) protected by dendrimer group into a 50mL two-necked flask, 0.08mmol tetrakis (triphenylphosphine) palladium (94mg), the system pumped N 2 Three times, then add 10 mL of degassed toluene, 2 mL of ethanol and 2 mol / L potassium carbonate solution under the protection of nitrogen, and then reflux at 110 ° C for 24 h, then cool the system to room temperature, filter (through diatomaceous earth) to obtain the filtrate, Spin-dried and separated by column chromatography to obtain 200 mg of a white solid with structural formula I-2, yield: 64%.
[0043] During the separation process of column chromatography, the silica gel used is 200-300 mesh...
Embodiment 3
[0050] A large sterically hindered alkoxyl substituted conjugated compound with triarylamine as the end group, the compound is bridged by phenylethynyl bridges with dendritic alkoxy groups, and its preparation method is as follows:
[0051] Add 1.71mmol 4-ethynyltriphenylamine (460mg), 2,5-bis(4-tert-butylbenzyloxy)-1,4-diiodobenzene (530mg, 0.81mmol), 0.17 mmol tetrakis (triphenylphosphine) palladium (94mg), 0.17mmol cuprous iodide (15mg), system pumping N 2 Three times, and then added 20 mL of degassed tetrahydrofuran and 10 mL of triethylamine under the protection of nitrogen, and then refluxed at 80 ° C for 24 h, the system was cooled to room temperature, filtered through diatomaceous earth to obtain the filtrate, spin-dried, and separated by column chromatography. 505 mg of a yellow solid of formula II-1 was obtained, yield: 67%.
[0052] During the separation process of column chromatography, the silica gel used is 200-300 mesh, and the eluent used is dichloromethane an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com