Charge transfer polymer
A charge transport, polymer technology, applied in the field of charge transport polymers, can solve the problems of toxicity, weak wear resistance, high risk and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
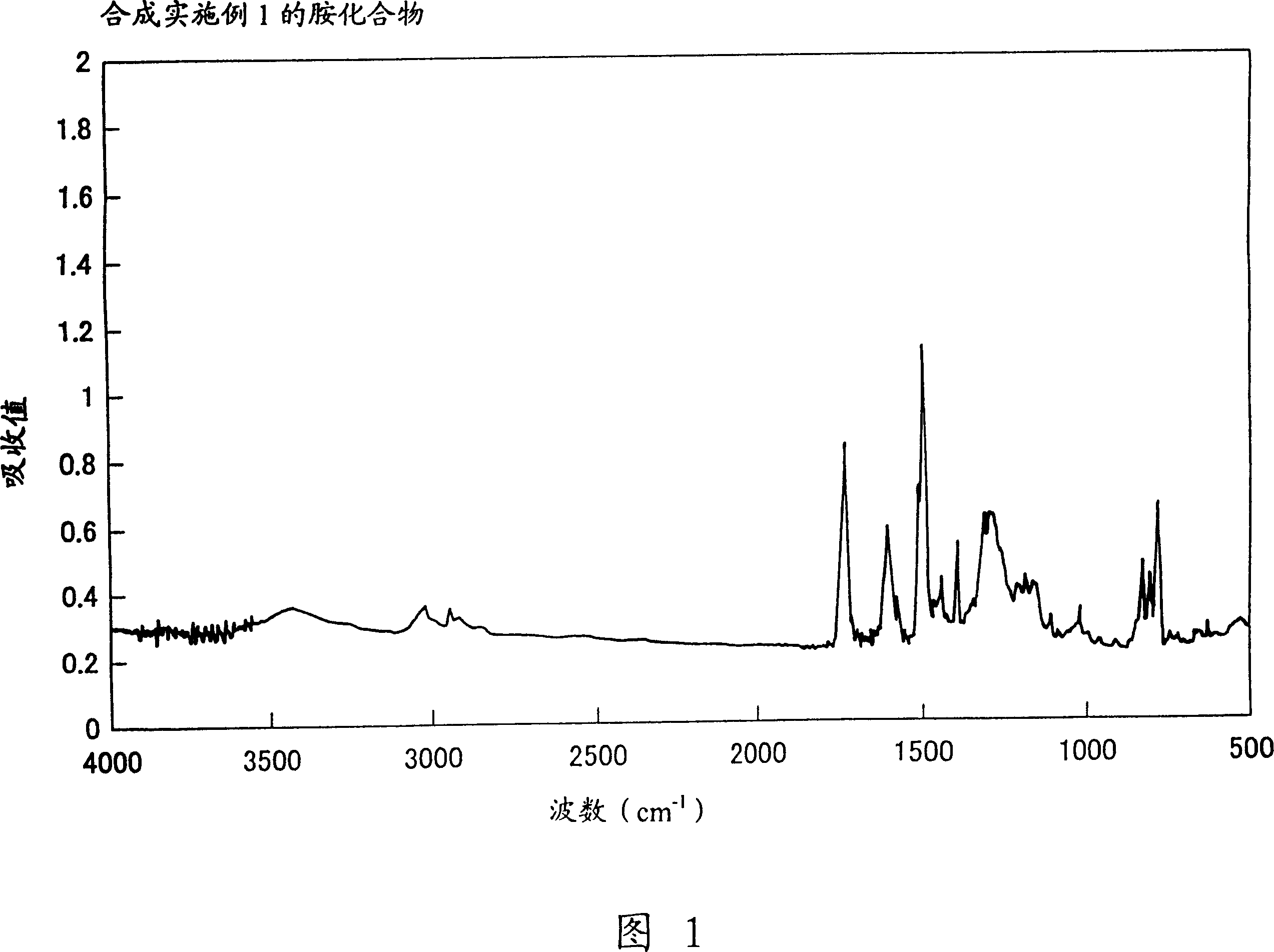
Embodiment 1
[0114] Synthesis of Charge Transport Polymers (17).
[0115] In a 50ml flask, put 1.0 g of N,N'-bis(1-naphthyl)-N,N'-bis[4-(2-methoxycarbonylethyl)phenyl]-[1,1'-bis phenyl]-4,4'-diamine, 2.0 g of ethylene glycol and 0.05 g of titanium tetrabutoxide, and the mixture was heated and stirred at 200° C. for 3 hours under a nitrogen stream. N,N'-bis(1-naphthyl)-N,N'-bis[4-(2-methoxycarbonylethyl)phenyl]-[1,1'-biphenyl]-4 was detected, After the 4'-diamine was consumed, the reaction system was heated at 200° C. while reducing the pressure in the reaction system to 0.5 mmHg (millimeter mercury column) to distill off ethylene glycol. In this way, the reaction was continued for 4 hours. Then, the reaction system was cooled to room temperature, and the product of the reaction was dissolved in 50 ml of toluene. The insoluble matter was filtered off with polytetrafluoroethylene (PTFE filter) having a mesh of 0.5 μm. The filtrate was added dropwise to 300ml of methanol with stirring. T...
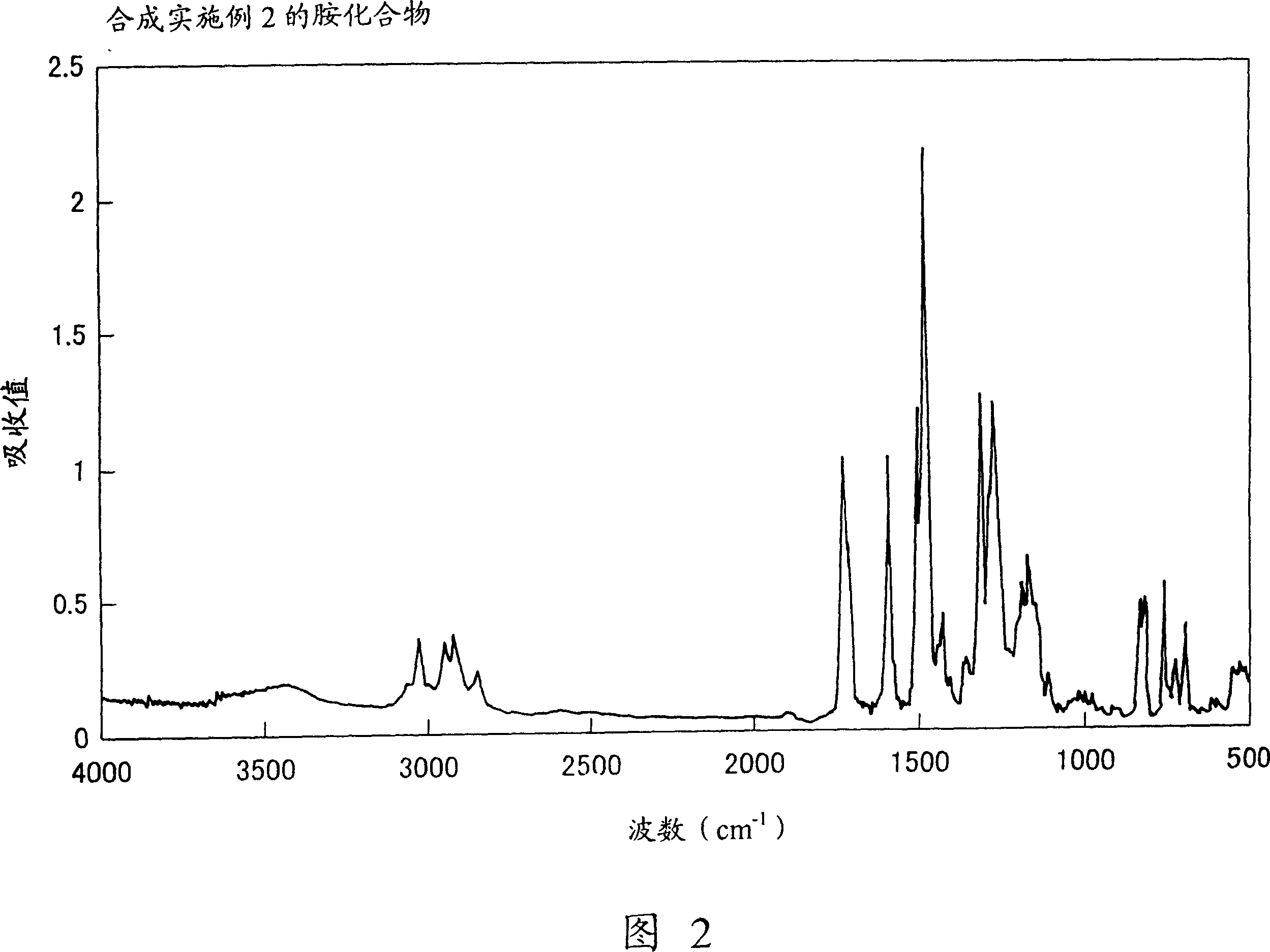
Embodiment 2
[0117] Synthesis of Charge Transport Polymer (32).
[0118] 1.0 grams of N, N'-bis[(4-phenyl)phenyl]-N,N'-bis[4-(2-methoxycarbonyl)phenyl]-[1,1'- biphenyl]-4,4'-diamine, 2.0 g of ethylene glycol and 0.05 g of titanium tetrabutoxide, and the mixture was heated and stirred at 200° C. for 3 hours under a nitrogen stream. N,N'-bis[(4-phenyl)phenyl]-N,N'-bis[4-(2-methoxycarbonyl)phenyl]-[1,1'-biphenyl]- After 4,4'-diamine was consumed, the reaction system was heated at 200°C while reducing the pressure in the reaction system to 0.5 mmHg to distill off ethylene glycol. In this way, the reaction was continued for 4 hours. Then, the reaction system was cooled to room temperature, and the reaction product was dissolved in 50 ml of toluene. The insoluble matter was filtered off with a PTFE filter having a mesh of 0.5 μm. The filtrate was added dropwise to 300ml of methanol with stirring. The polymer is precipitated in this manner. The resulting polymer was filtered off, washed wel...
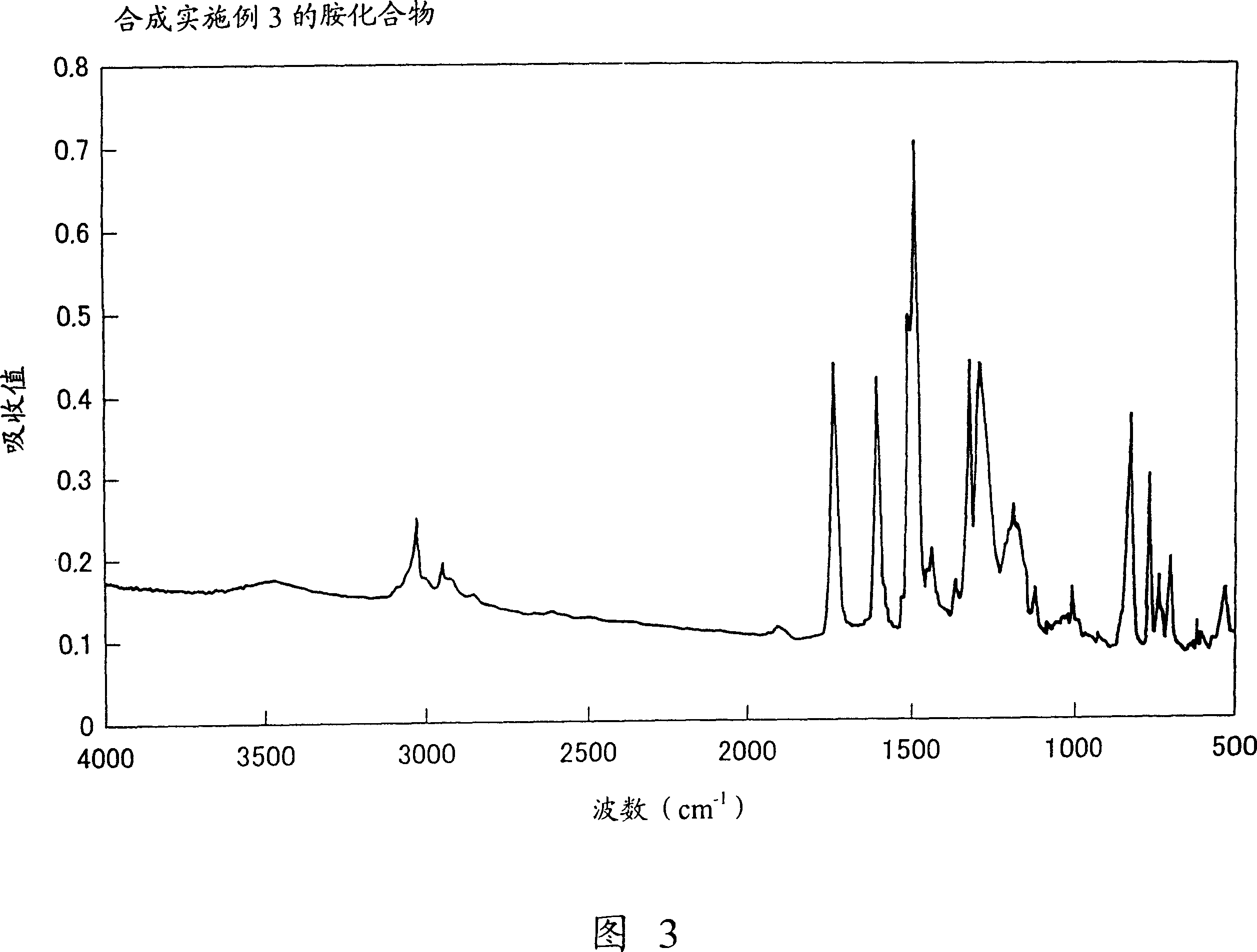
Embodiment 3
[0120] Synthesis of Charge Transport Polymers (36).
[0121] In a 50ml flask, drop 1.0 g of N, N'-bis[(4-biphenyl)phenyl]-N,N'-bis[4-(2-methoxycarbonylethyl)phenyl]-[1 ,1′-biphenyl]-4,4′-diamine, 2.0 g of ethylene glycol and 0.05 g of titanium tetrabutoxide, and the mixture was heated and stirred at 200° C. for 3 hours under a nitrogen stream. N,N'-bis[(4-biphenyl)phenyl]-N,N'-bis[4-(2-methoxycarbonylethyl)phenyl]-[1,1'-biphenyl] detected After the base]-4,4'-diamine was consumed, the reaction system was heated at 200°C while reducing the pressure in the reaction system to 0.5 mmHg to distill off ethylene glycol. In this way, the reaction was continued for 4 hours. Then, the reaction system was cooled to room temperature, and the reaction product was dissolved in 50 ml of toluene. The insoluble matter was filtered off with a PTFE filter having a mesh of 0.5 μm. The filtrate was added dropwise to 300ml of methanol with stirring. The polymer is precipitated in this manner. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com