A kind of conjugated ligand bridged diarylamine and ruthenium acetylene terminal compound and application thereof
A technology of diarylamines and compounds, applied in the field of organic synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
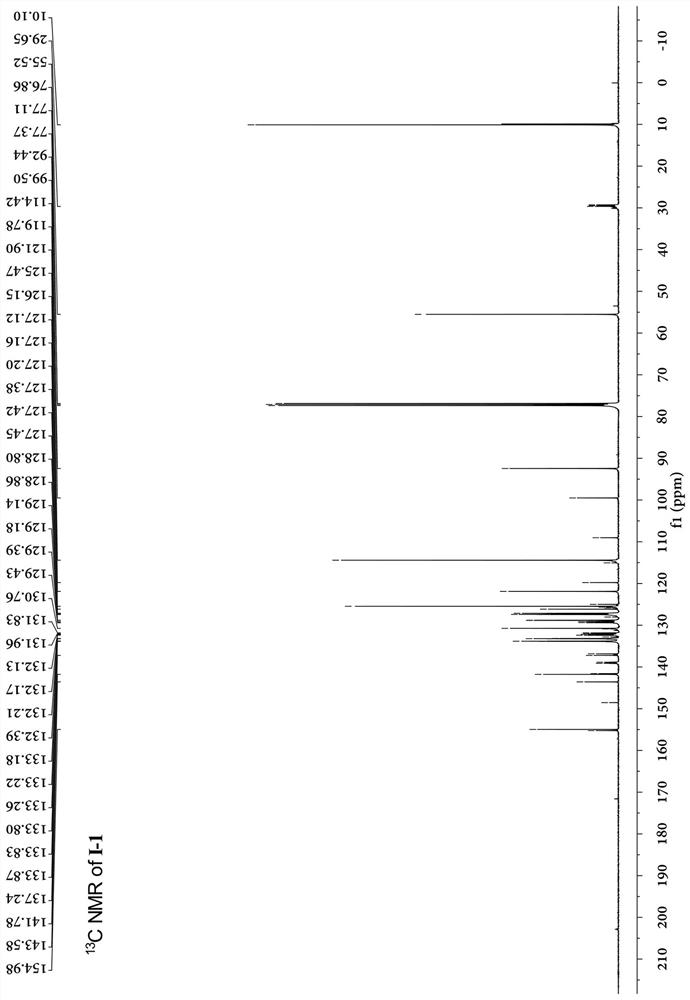
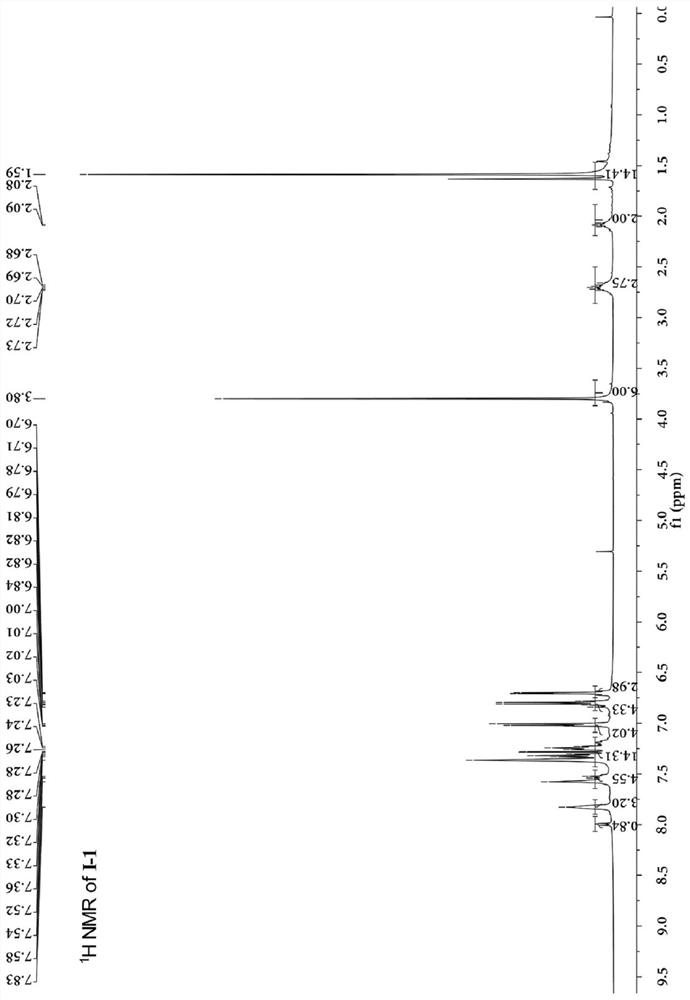
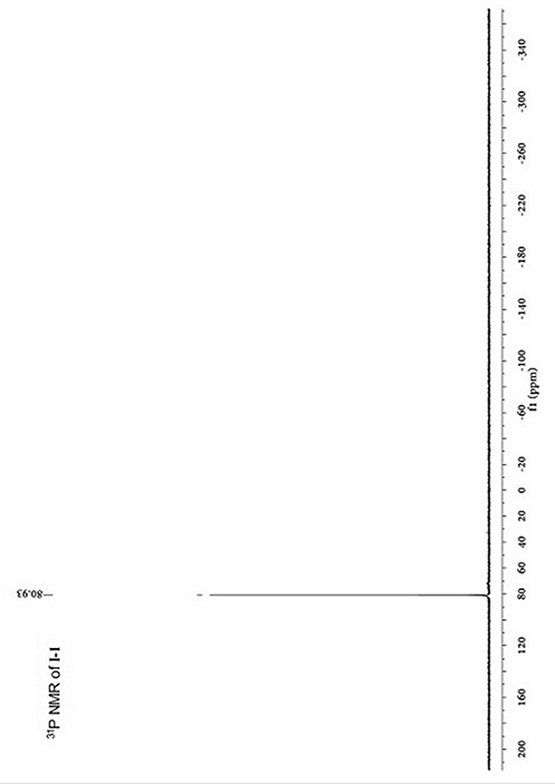
[0053]A kind of conjugated ligand bridged diarylamine and ruthenium acetylene terminal compound, its structural formula I-1 is as follows:
[0054]
[0055] The method for preparing the diarylamine and ruthenium acetylene terminal compound bridged by the conjugated ligand comprises the following steps:
[0056] a. Under the protection of nitrogen, 0.3mmol (1,2-bisdiphenylphosphinoethane) ruthenium chloride Cp * Ru(dppe)Cl, 0.25mmol 4-trimethylsilylethynyl-N,N-bis(4-methoxyphenyl)-1-aniline) and 2mmol potassium fluoride were dissolved in 20ml methanol and 3-4ml In the mixed solution of tetrahydrofuran, cool to room temperature after heating to reflux for 24h;
[0057] b. suction filtration, the resulting solids were washed with 10ml of methanol and 10ml of n-hexane, and then recrystallized with dichloromethane and n-hexane;
[0058] c. Acetone and petroleum ether with a volume ratio of 1:2 were selected as eluents for column chromatography to obtain 150 mg of yellow crysta...
Embodiment 2
[0065] A kind of conjugated ligand bridged diarylamine and ruthenium acetylene terminal compound, its structural formula I-2 is as follows:
[0066]
[0067] The method for preparing the diarylamine and ruthenium acetylene terminal compound bridged by the conjugated ligand comprises the following steps:
[0068] a. Under nitrogen protection, 0.3 mmol pentamethylcyclopentadienyl (1,2-bisdiphenylphosphine ethane) ruthenium chloride Cp * Ru(dppe)Cl, 0.25mmol 3-trimethylsilylethynyl-N,N-di(4-methoxyphenyl)-1-aniline) and 2mmol potassium fluoride were dissolved in 20ml methanol and 3-4ml In the mixed solution of tetrahydrofuran, cool to room temperature after heating to reflux for 24h;
[0069] b. suction filtration, the resulting solids were washed with 10ml of methanol and 10ml of n-hexane, and then recrystallized with dichloromethane and n-hexane;
[0070] c. Acetone and petroleum ether with a volume ratio of 1:2 were selected as eluents for column chromatography separation...
Embodiment 3
[0077] A diarylamine and ruthenium acetylene terminal compound bridged by a conjugated ligand, its structural formula I-3 is as follows:
[0078]
[0079] The method for preparing the diarylamine and ruthenium acetylene terminal compound bridged by the conjugated ligand comprises the following steps:
[0080] a. 0.3mmol pentamethylcyclopentadienyl (1,2-bisdiphenylphosphine ethane) ruthenium chloride Cp * Dissolve Ru(dppe)Cl, 0.25mmol 4-trimethylsilylethynyl-N,N-bis(4-methoxyphenyl)-1-naphthylamine) and 2mmol potassium fluoride in 20ml methanol and 2ml tetrahydrofuran In the mixed solution, cool to room temperature after heating to reflux for 24h;
[0081] b. Suction filtration, the obtained solid was washed with 10ml of methanol and 10ml of n-hexane respectively, and then recrystallized with dichloromethane and n-hexane to obtain 180mg of a tea-green solid, yield: 68%.
[0082] The carbon nuclear magnetic resonance spectrum, the proton nuclear magnetic resonance spectrum ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com