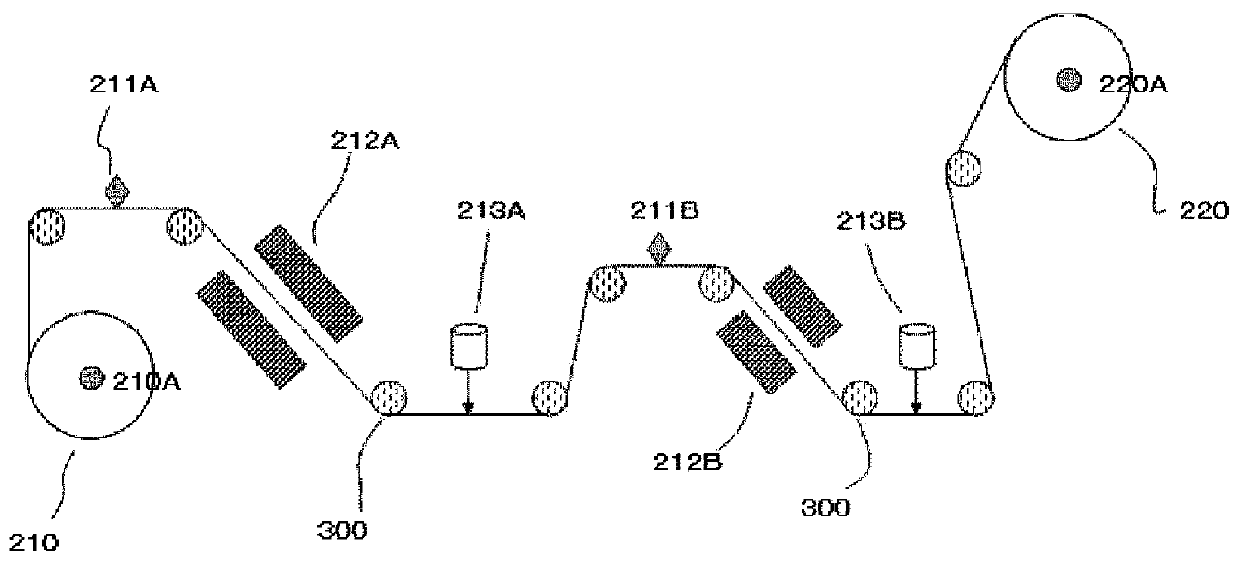
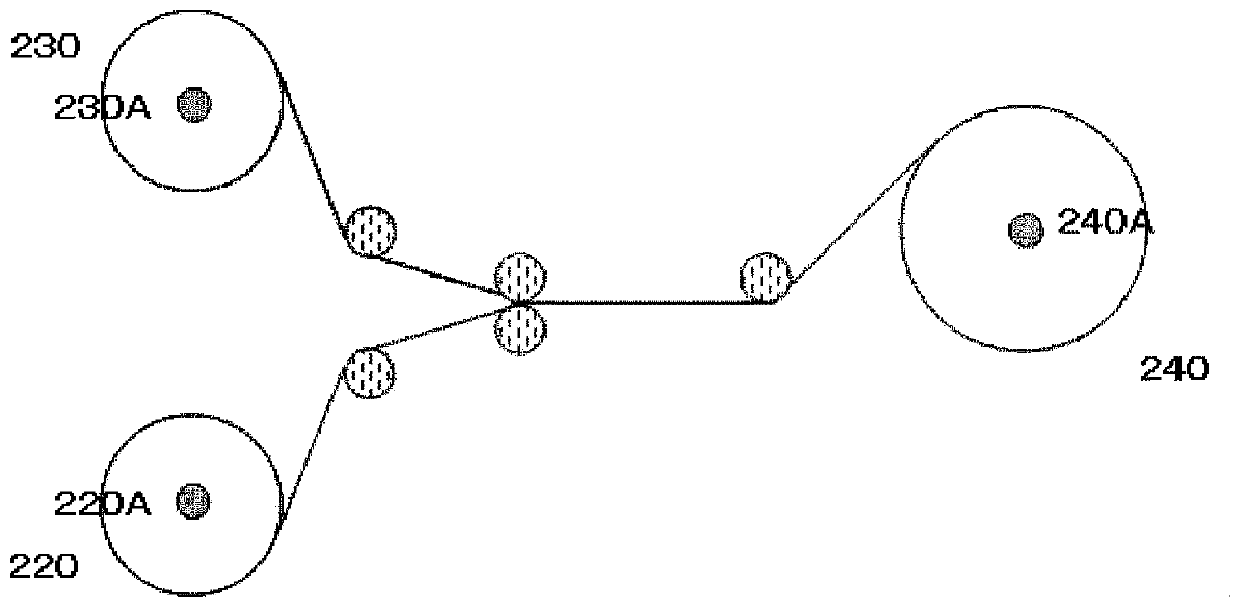
Compounds and Compositions
A technology of compound and composition, applied in the field of compound and composition, can solve the problem of low dichroic ratio of polarizing film
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0313]
[0314] 1.00 g of the compound represented by the formula (2A), 4.00 g of water and 1.30 g of 35% hydrochloric acid were mixed. After cooling the obtained mixture to 0° C., 0.74 g of a 33% sodium nitrite aqueous solution was added dropwise. After stirring the obtained mixture for 30 minutes, 0.03 g of sulfamic acid was added. The obtained mixture was added dropwise to a mixture consisting of 0.74 g of the compound represented by the formula (3), 1.06 g of sodium acetate, and 7.44 g of water at 0°C. The precipitate was filtered and washed 3 times with water.
[0315] 0.32 g of sodium hydroxide and 8.00 g of water were added to the obtained precipitate. The obtained mixture was stirred at 100°C for 2 hours, and then cooled to 10°C. The precipitate was filtered and washed with water. The obtained solid was purified by silica gel column chromatography (eluent: toluene) after drying. The obtained solid was washed with acetonitrile and then dried to obtain 0.12 g of ...
Embodiment 2
[0321]
[0322] In Example 1, except that the compound represented by the formula (2B) was used instead of the compound represented by the formula (2A), it was carried out in the same manner as in Example 1, and the compound represented by the formula (1B) was obtained as an orange solid. (hereinafter referred to as compound (1B)) 0.020 g.
[0323] Mw: 399 (LC-MS)
[0324] Maximum Absorption Wavelength (λ max2 )=389nm (chloroform solution)
Embodiment 3
[0326]
[0327] In Example 1, except that the compound represented by the formula (2C) was used instead of the compound represented by the formula (2A), it was carried out in the same manner as in Example 1, and the compound represented by the formula (1C) was obtained as an orange solid. (hereinafter referred to as compound (1C)) 0.009 g.
[0328] Mw: 400 (LC-MS)
[0329] Maximum Absorption Wavelength (λ max2 )=399nm (chloroform solution)
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com