Method of simple catalysis to prepare spiro-1,3-dioxoindanpyrane derivative and catalyst for preparing same
A technology of pyran derivatives and dioxindene, which is applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. Large usage, not easy to biodegrade and other problems, to achieve the effect of more recycling times, less environmental hazards, and easy promotion and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] For the preparation method of the above-mentioned catalyst used in the present invention, see the related literature (Introduction of a novel basic ionic liquid containing dual basic functional groups for the efficient synthesis of spiro-4H-pyrans[J], Journal of Molecular Liquids, 2016, 224: 1092 ~ 1101 ).
[0033] The specific process of the method for preparing spirocyclic 1,3-dioxoindanpyran derivatives in the present invention is: adding ninhydrin, malononitrile, 1,3-cyclohexanedione compound and catalyst to ethanol respectively In the aqueous solution, and mixed uniformly, the molar ratio of ninhydrin (1), malononitrile (2) and 1,3-cyclohexanedione compound (3) in the above reaction raw materials is 1:(1~1.2):1 , The 1,3-cyclohexanedione compound is 1,3-cyclohexanedione, 5,5-dimethyl-1,3-cyclohexanedione, barbituric acid, 1,3-dimethyl Any one of ylbarbituric acid, 4-hydroxycoumarin and 8-hydroxyquinoline; the molar amount of the catalyst is 3 to 6% of the molar amoun...
Embodiment 1
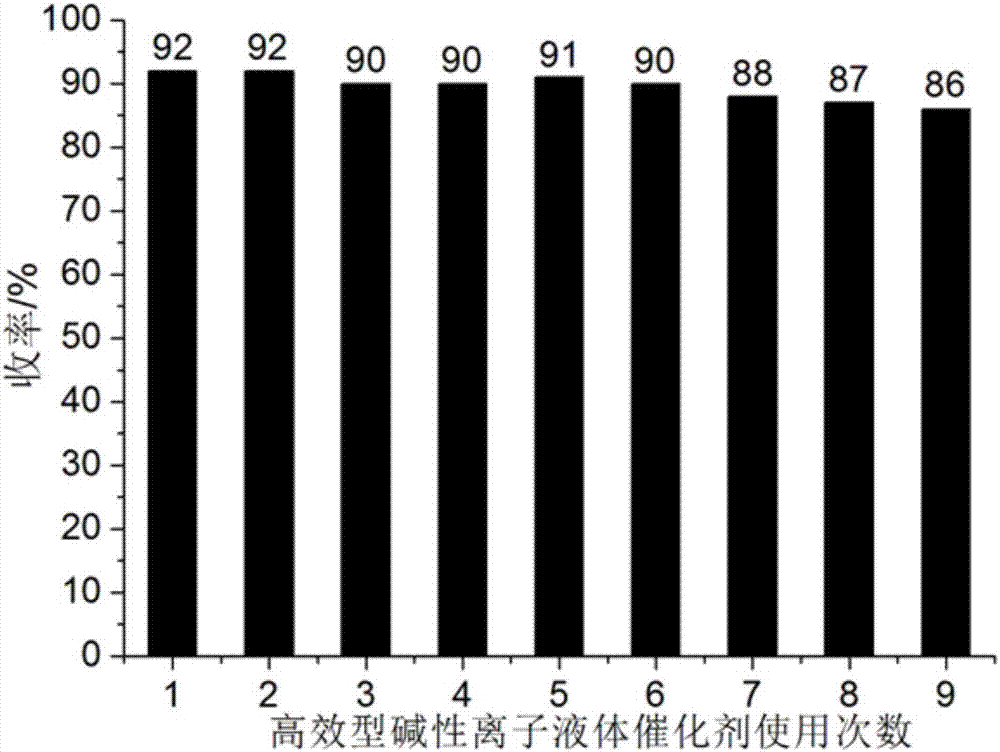
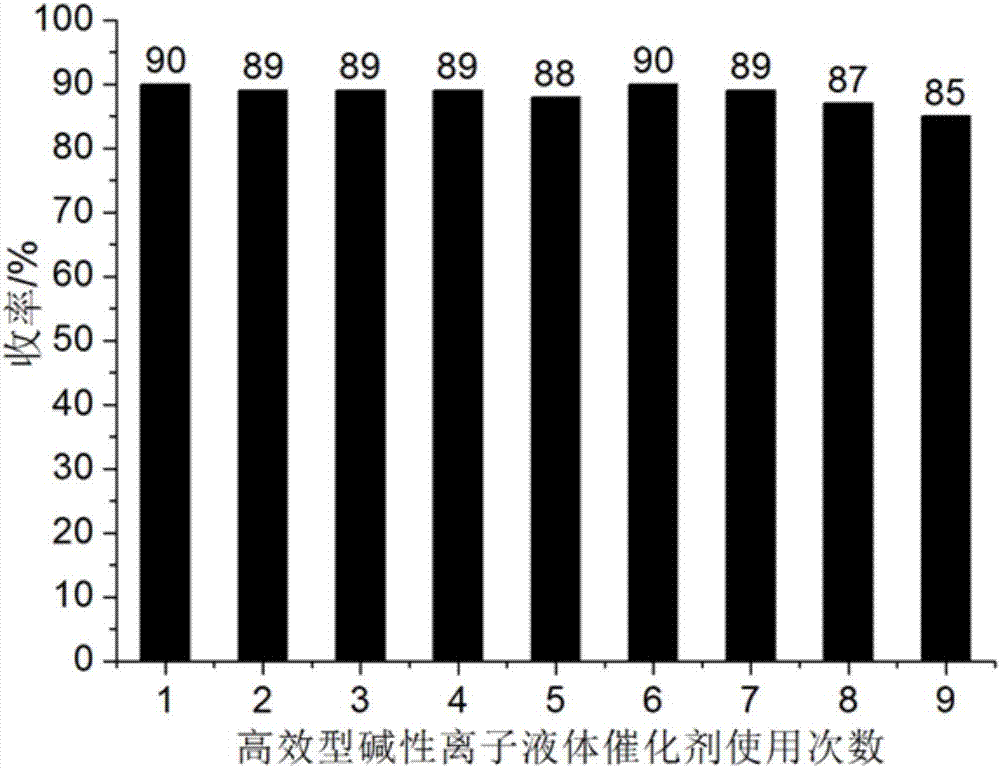
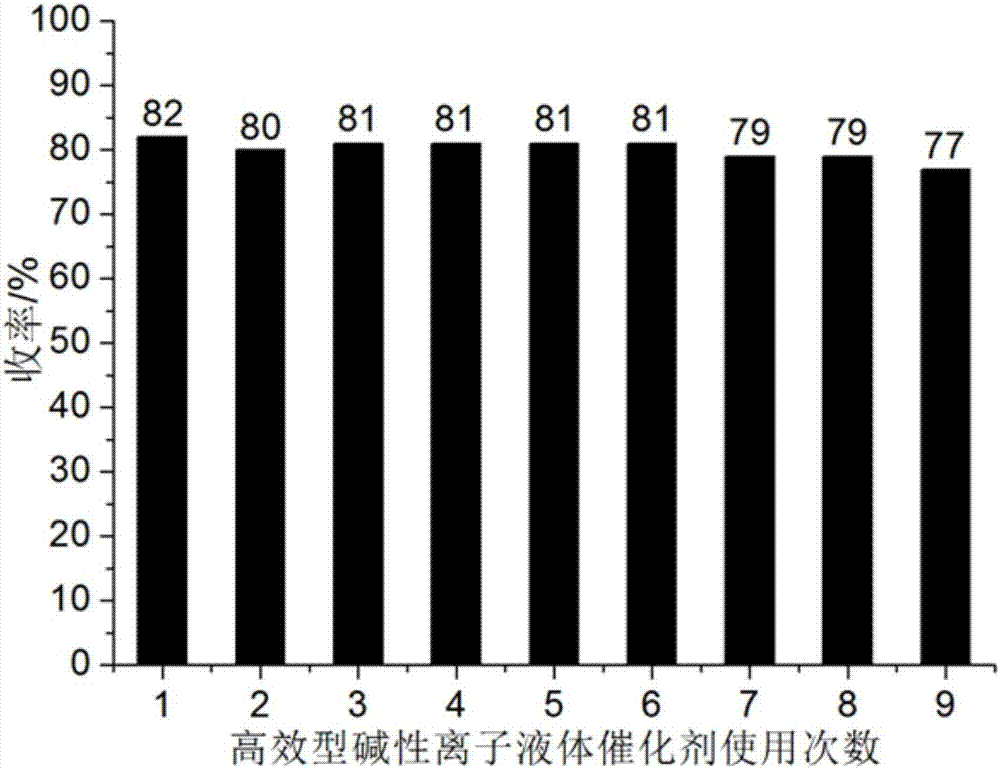
[0040] Add 1mmol ninhydrin, 1mmol malononitrile, 1mmol 1,3-cyclohexanedione and 0.04mmol catalyst respectively into a 50ml single-necked flask with a stir bar and a condenser containing 4ml 92% ethanol aqueous solution. React at ℃ for 10 minutes, the reaction pressure is one atmosphere, and perform TLC (Thin Plate Chromatography) tracking. After the reaction is completed, cool to room temperature, crush the precipitated solid, stand, and filter with suction. The filter residue is washed with 92% ethanol aqueous solution and dried in vacuo Obtain 2'-amino-3'-cyano-1,3,5'-trioxo-5',6',7',8'-tetrahydrospirocyclic indanpyranobenzene, the yield is 92%, the filtrate after 4ml was made up with washing liquid, and ninhydrin, malononitrile and 1,3-cyclohexanedione were directly added for repeated use.
[0041] The 2'-amino-3'-cyano-1,3,5'-trioxo-5',6',7',8'-tetrahydrospirocyclic indane pyranobenzene obtained in this example The performance parameters are as follows: IR (KBr): 3402, 3315,...
Embodiment 2~4
[0044] A different reaction temperature (shown in Table 1) was adopted to replace the reaction temperature described in Example 1. Other conditions were the same as in Example 1. The experimental results are shown in Table 1, and the 2'-amino-3' obtained in Examples 2 to 4 The performance parameters of -cyano-1,3,5'-trioxo-5',6',7',8'-tetrahydrospiroindanpyranobenzene are similar to those of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com