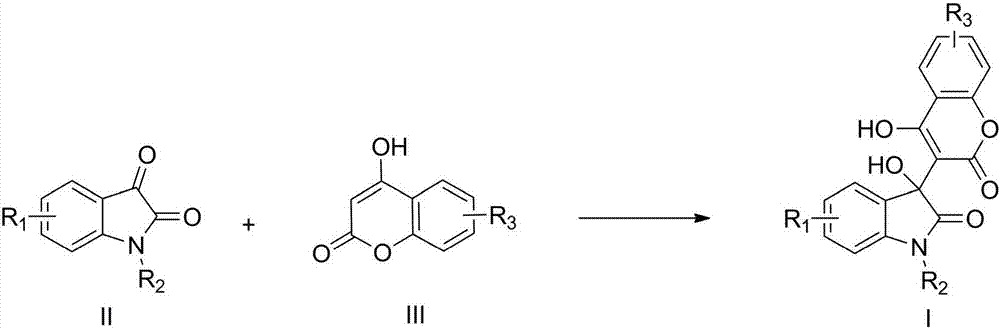
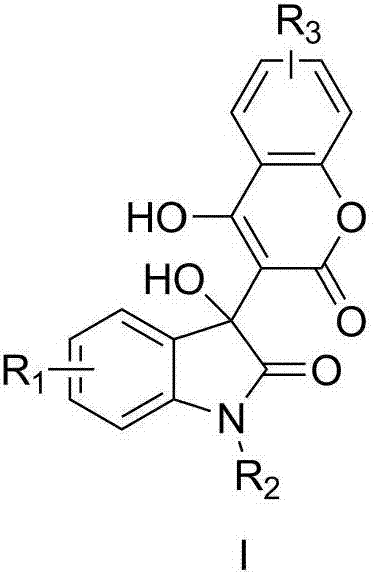
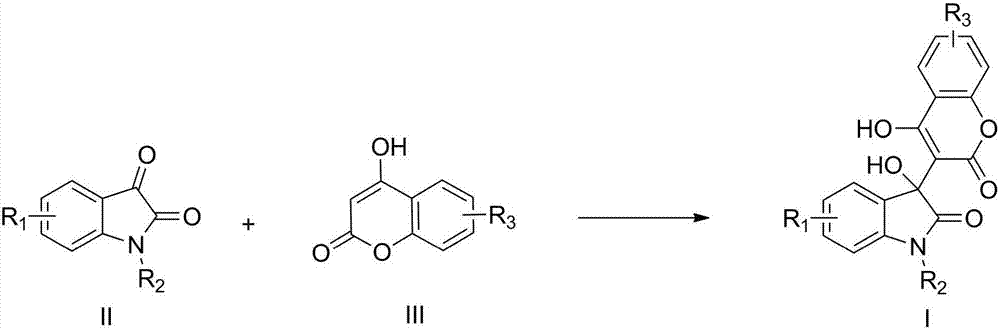
Preparation method of 3-hydroxy-3-(4-hydroxy-2-oxo-2-hydrogen-chromene-3-yl)indolin-2-one
An indoline and hydroxyl technology, applied in the field of organic compound synthesis, can solve problems such as complicated operation and complicated reaction apparatus, and achieve the effects of simple operation, high yield and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Preparation of 3-hydroxyl-3-(4-hydroxyl-2-oxo-2-hydrogen-chromene-3-yl)-5-fluoroindoline-2-one (I-1)
[0018] Add 5-fluoroisatin (1mmol), 4-hydroxycoumarin (1.05mmol), tetrabutylammonium bromide (0.1mmol) and water (4mL) into a microwave reaction tube, microwave power 400W, and irradiate at 70°C for 10min , stop the microwave radiation, cool to room temperature, filter under reduced pressure, wash the filter cake with ice ethanol (0.5ml), and dry under reduced pressure to obtain a white solid with a yield of 96%.
[0019] 1 H NMR (CDCl 3 )δ: 10.48(s, 1H), 7.92(d, 1H, J=8.0Hz), 7.68~7.63(m, 2H), 7.43~7.36 (m, 3H), 7.16(d, 1H, J=8.0Hz ),7.08~7.03(m,1H),6.83~6.80(m,1H); 13 C NMR (CDCl 3 )δ: 176.07, 165.55, 160.07, 157.25, 152.69, 139.56, 133.39, 133.15, 132.85, 132.78, 124.82, 116.30, 116.17, 110.91, 100.66, 91.43.
Embodiment 2
[0020] Example 2: Preparation of 3-hydroxyl-3-(4-hydroxyl-2-oxo-2-hydrogen-chromen-3-yl)-7-fluoroindoline-2-one (I-2)
[0021] Add 7-fluoroisatin (1 mmol), 4-hydroxycoumarin (1.1 mmol), tetrabutylammonium chloride (0.1 mmol) and water (4 mL) into a microwave reaction tube, microwave power 400W, and irradiate at 60°C for 15 min , stop the microwave radiation, cool to room temperature, filter under reduced pressure, wash the filter cake with ice ethanol (0.5ml), and dry under reduced pressure to obtain a white solid with a yield of 94%.
[0022] 1 H NMR (CDCl 3 )δ:10.99(s,1H),7.92(d,1H,J=8.0Hz),7.69~7,65(m,2H),7.43~7.34(m,3H),7.19~7.14(m,1H) ,7.11~7.09(m,1H),6.94~6.91(m,1H); 13 C NMR (CDCl 3 )δ: 175.83, 165.39, 160.10, 152.65, 133.48, 124.91, 124.36, 123.02, 120.37, 117.49, 117.32, 116.81, 100.87, 91.44, 78.65.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com