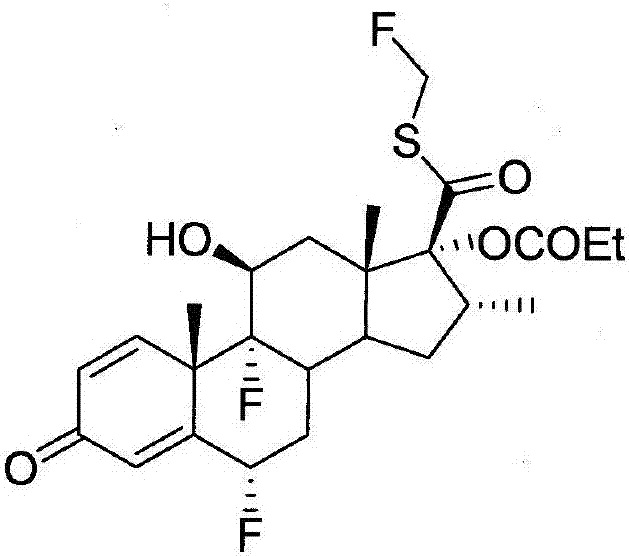
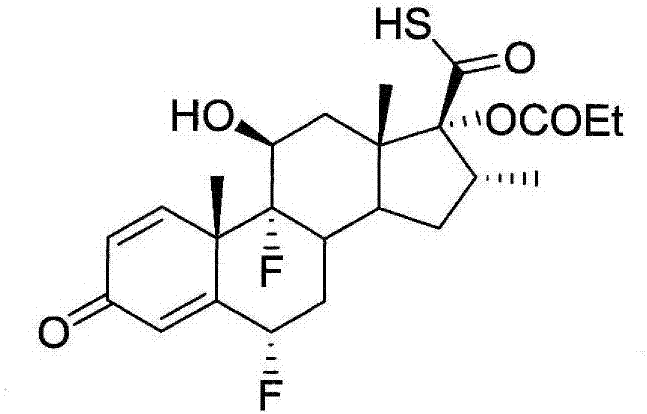
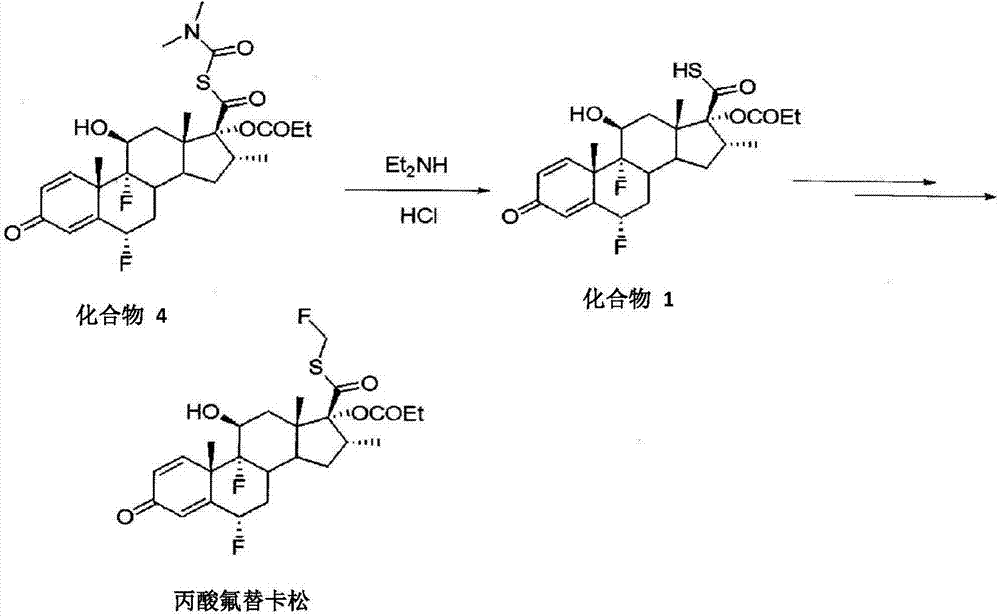
Methods of preparing intermediate of fluticasone propionate
A technology of fluticasone propionate thioacid and intermediates, which is applied in the field of preparation of fluticasone propionate thioacid intermediates, and can solve the problems that impurities are difficult to be removed, affected, and reduce the quality of the final product.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045]According to an embodiment of the present invention, the preparation method of the fluticasone propionate thioacid intermediate comprises: processing 17.-[( N,N-dimethylformamide)mercapto]carbonyl compounds (such as compounds 4,17.-[(N,N-dimethylformamide)mercapto]carbonyl-6.,9.-difluoro-11. -Hydroxy-16.-methyl-3-oxo-17.-oxopropoxyandrost-1,4-diene or its derivatives), the amide from the 17.-[(N,N- Cleavage from dimethylformamide) mercapto] carbonyl compounds; processing the solution to separate the water-soluble fraction; and adding acid to the water-soluble fraction to obtain fluticasone propionate thioacid intermediates (such as compounds 1, 6., 9.-Difluoro-11.-hydroxy-16.-methyl-3-oxo-17.-oxopropoxyandrost-1,4-diene-17.-thioformate or its derivatives things). The amide can be cleaved from the 17.-[(N,N-dimethylformamide)mercapto]carbonyl compound by hydrolysis. The amide may comprise, for example, (CH 3 )2NCOOH. Treatment of the solution removes impurities follo...
example 1
[0065] Compound 2, 6.,9.-Difluoro-11.-hydroxy-16.-methyl-3-oxoandrost-1,4-diene-17.-carboxylic acid, was prepared as follows.
[0066] 36.2 g of periodic acid was dissolved in 72 ml of water to obtain a periodic acid solution. The periodic acid solution was added dropwise to a stirred suspension composed of 30 g of fluticasone and 150 ml of tetrahydrofuran at a temperature of 0° C. to 10° C. to obtain a reaction solution. After the dropwise addition (or approximately) of the periodic acid solution was completed, the reaction solution was continued to be stirred for 2 hours. The THF was then distilled off (at least most of the THF was distilled off), cooled to 0°C to 10°C and held in this temperature range for 2 hours, then filtered to obtain the filtered product. The filtered product was washed with water and dried at 50° C. to obtain 28.3 g of a final product with a purity of 99.5% (ie, the final product contained 99.5 wt% of compound 2 based on the total mass of the final p...
example 2
[0071] Compound 3, 6.,9.-Difluoro-11.-hydroxy-16.-methyl-3-oxo-17.-oxopropoxyandrost-1,4-diene-17, was prepared as follows .-carboxylic acid.
[0072] At a temperature of 0° C. to 10° C., 40 ml of triethylamine was added to the suspension containing compound 2 (composed of 50 g of compound 2 with a purity of 99.5 wt % and 250 ml of ethyl ketone) to obtain a reaction solution. Then 45.6 ml of propionyl chloride was added to the reaction solution. After the addition was complete (or substantially complete), the reaction solution was stirred for 1 hour. Then 50ml of diethylamine was added, and stirring was continued for 2 hours to obtain a reaction mixture. Afterwards, 2M hydrochloric acid was added to acidify the reaction mixture to a pH of about 1 to 2 and to produce a precipitated product. The precipitated product was filtered, washed with water and dried at 50° C. to obtain 54.5 g of a final product with a purity of 99.2% (that is, the final product contained 99.2 wt% of c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com