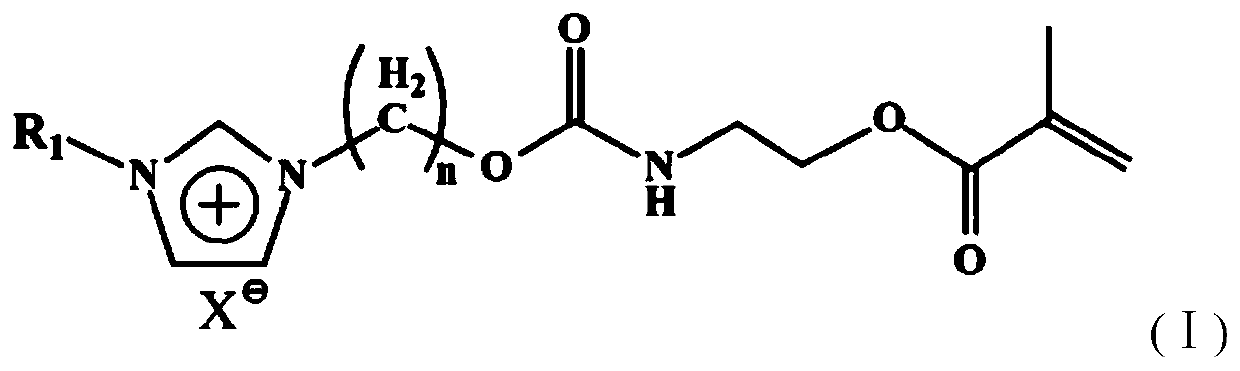
Antibacterial polymerizable monomer containing halide imidazolium salt structure and its preparation method and application
A technology of imidazolium halide and polymerized monomers, which is applied in the field of polymerizable monomers, can solve the problems of unsustainable antibacterial properties, drug resistance of bacteria, and decreased mechanical properties of bone cement, etc., and achieve the effect of broad-spectrum and high-efficiency antibacterial properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
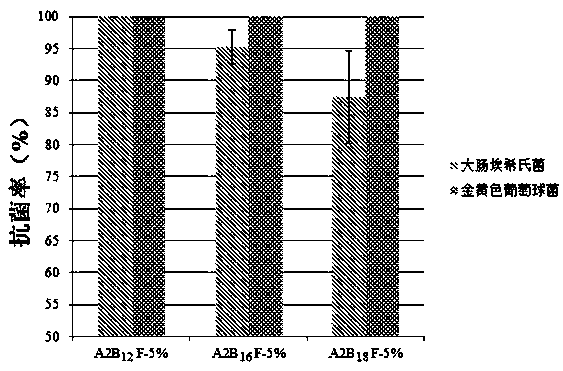
[0036] This embodiment prepares A 2 B 12 F monomer, the main steps are as follows:
[0037] Add 1.12g of N-hydroxyethylimidazole and 50mL of tetrahydrofuran into the reaction vessel, add 1.55g of isocyanoethyl methacrylate dropwise at a rate of 20 drops per minute under stirring, and react at 45°C for 12 hours , to completely react the isocyanate group, then adjust the reaction temperature to 65°C, add 2.49g bromododecane, react for 72 hours, and generate the product, and then perform purification treatment to obtain A 2 B 12 Type F monomer, the product is characterized by H NMR spectrum:
[0038] 1H-NMR (DMSO-d6, 600MHz): δ0.85[3H, t], 1.24[18H, s], 1.79[2H, m], 1.87[3H, s], 3.24[2H, q], 4.07[ 2H,t], 4.20[2H,t], 4.32[2H,t], 4.45[2H,t], 5.68[1H,s], 6.05[1H,s], 7.52[1H,t], 7.80[1H ,s], 7.85[1H,s], 9.29[1H,s]
Embodiment 2
[0040] This embodiment prepares A 2 B 16 F monomer, the main steps are as follows:
[0041]Add 1.12g of N-hydroxyethylimidazole and 50mL of tetrahydrofuran into the reaction vessel, add 1.55g of isocyanoethyl methacrylate dropwise at a rate of 20 drops per minute under stirring, and react at 45°C for 12 hours , to completely react the isocyanate group, then adjust the reaction temperature to 65°C, add 3.05g of hexadecane bromide, and react for 72 hours to generate the product, which is then purified to obtain A 2 B 16 Type F monomer, the product is characterized by H NMR spectrum:
[0042] 1H-NMR (DMSO-d6, 600MHz): δ0.85[3H, t], 1.23[26H, s], 1.77[2H, m], 1.87[3H, s], 3.24[2H, q], 4.07[ 2H,t], 4.19[2H,t], 4.32[2H,t], 4.44[2H,t], 5.68[1H,s], 6.04[1H,s], 7.49[1H,t], 7.79[1H ,s], 7.84[1H,s], 9.26[1H,s]
Embodiment 3
[0044] This embodiment prepares A 2 B 18 F monomer, the main steps are as follows:
[0045] Add 1.12g of N-hydroxyethylimidazole and 50mL of tetrahydrofuran into the reaction vessel, add 1.55g of isocyanoethyl methacrylate dropwise at a rate of 20 drops per minute under stirring, and react at 45°C for 12 hours , to completely react the isocyanate group, then adjust the reaction temperature to 65°C, add 3.33g bromododecane, react for 72 hours, and generate the product, and then perform purification treatment to obtain A 2 B 18 Type F monomer, the product is characterized by H NMR spectrum:
[0046] 1H-NMR (DMSO-d6, 600MHz): δ0.85[3H, t], 1.24[30H, s], 1.79[2H, m], 1.87[3H, s], 3.25[2H, q], 4.07[ 2H,t], 4.20[2H,t], 4.32[2H,t], 4.45[2H,t], 5.68[1H,s], 6.05[1H,s], 7.51[1H,t], 7.80[1H ,s], 7.85[1H,s], 9.30[1H,s]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com