Medicine for treating chronic glomerulonephritis and nephrotic syndrome and preparation method thereof
A technology for glomerulonephritis and nephrotic syndrome, applied in the field of medicine, can solve the problems of side effects, limited effect, and different efficacy of nephrotic syndrome, and achieve the effect of reducing proteinuria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
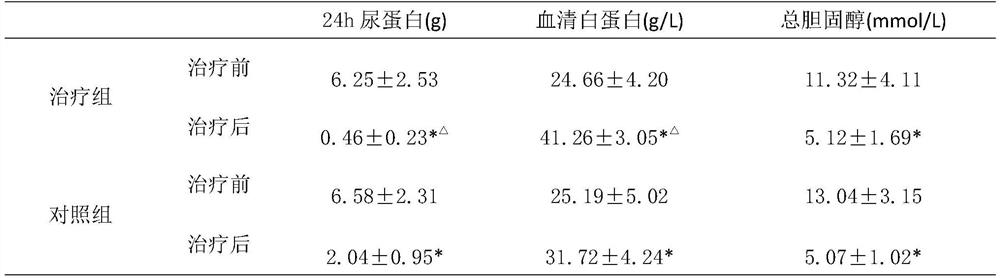
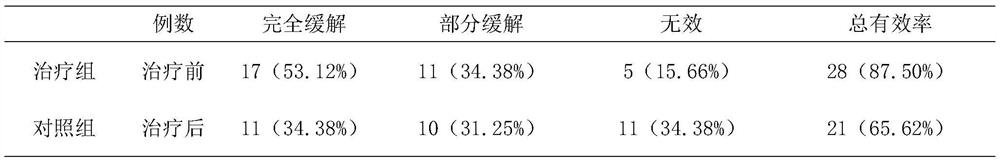
[0044] Formula: Astragalus 25kg, Dijincao 17kg, Codonopsis 15kg, Cornus 15kg, Rehmannia glutinosa 17kg, Atractylodes macrocephala 13kg, Poria cocos 13kg and Alisma 13kg.
[0045] Process: Take the above medicine, add 9 times the amount of water to soak for 13 minutes, first decoct on high heat for 13 minutes, then continue to decoct on low heat for 20 minutes, decoct 3 times, filter, combine the filtrate, and the filtrate is concentrated to 50-60 ° C. For the extract with a density of 1.20-1.25, take 30% starch and 0.7% magnesium stearate to mix with the extract, granulate with 60% ethanol, dry, crush, and fill capsules to obtain capsules agent.
[0046] Specification: 0.5g / grain;
[0047] Usage and dosage: 2-3 times a day, 2-3 capsules each time.
Embodiment 2
[0049] Formula: Astragalus 27kg, Dijincao 19kg, Codonopsis 17kg, Cornus 17kg, Rehmannia glutinosa 19kg, Atractylodes macrocephala 14kg, Poria cocos 14kg and Alisma 14kg.
[0050] Process: Take the above-mentioned medicine, add 9 times the amount of water to soak for 13 minutes, first decoct on high heat for 13 minutes until boiling, then continue to decoct on low heat for 20 minutes, filter, add 5 times the amount of water to the filter residue and decoct twice, each time After 30 minutes, filter and combine the filtrates to obtain the decoction.
[0051] Usage and dosage: 2-3 times a day, 100mL each time.
Embodiment 3
[0053] Formula: Astragalus 23kg, Dijincao 16kg, Codonopsis 13kg, Cornus 13kg, Rehmannia glutinosa 16kg, Atractylodes macrocephala 12kg, Poria cocos 12kg and Alisma 12kg.
[0054] Process: Take the above medicine, add 8-10 times the amount of water to soak for 10-15 minutes, first decoct on high heat for 15 minutes, then continue to decoct on low heat for 25 minutes, decoct 3 times, filter, combine the filtrate, and concentrate the filtrate to 50- The extract with a relative density of 1.20-1.25 at 60°C is mixed with 20% starch in the extract, pressed into tablets, and coated with a film to obtain tablets.
[0055] Specification: 0.5g / piece
[0056] Usage and dosage: 2-3 times a day, 2-3 tablets each time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com