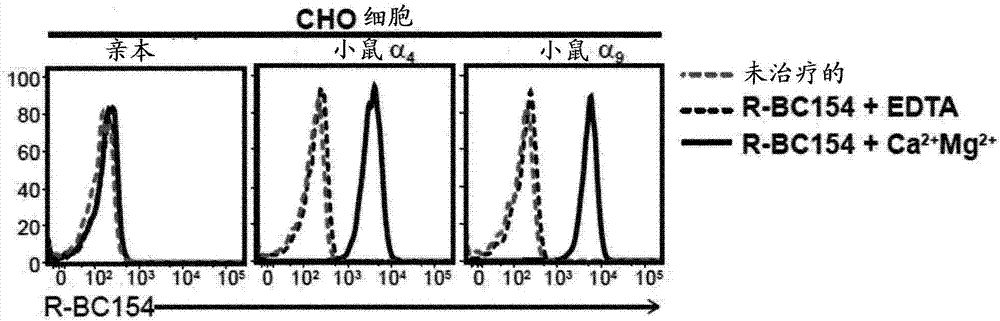
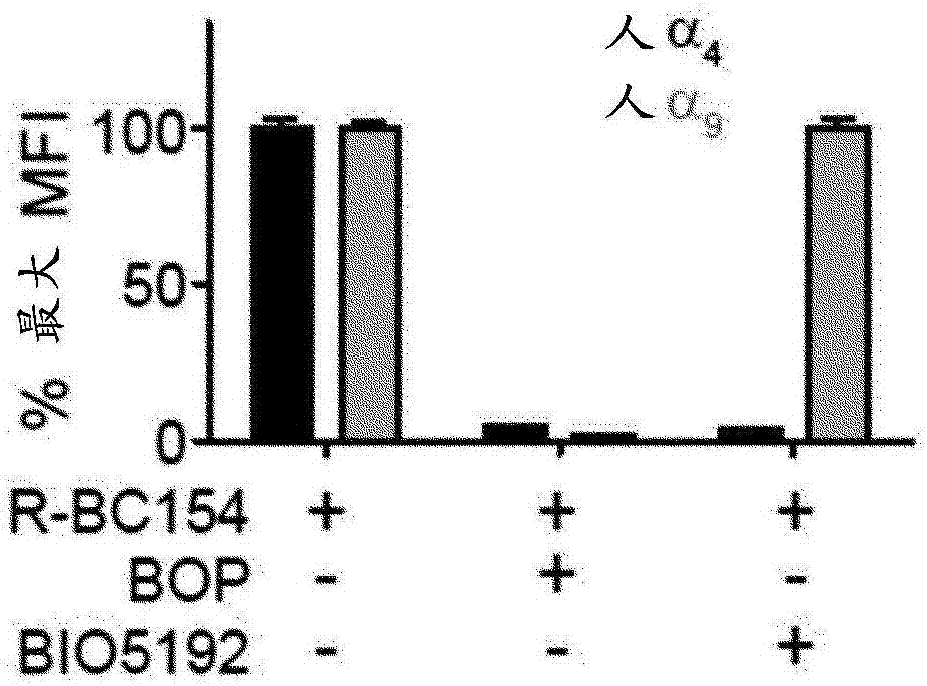
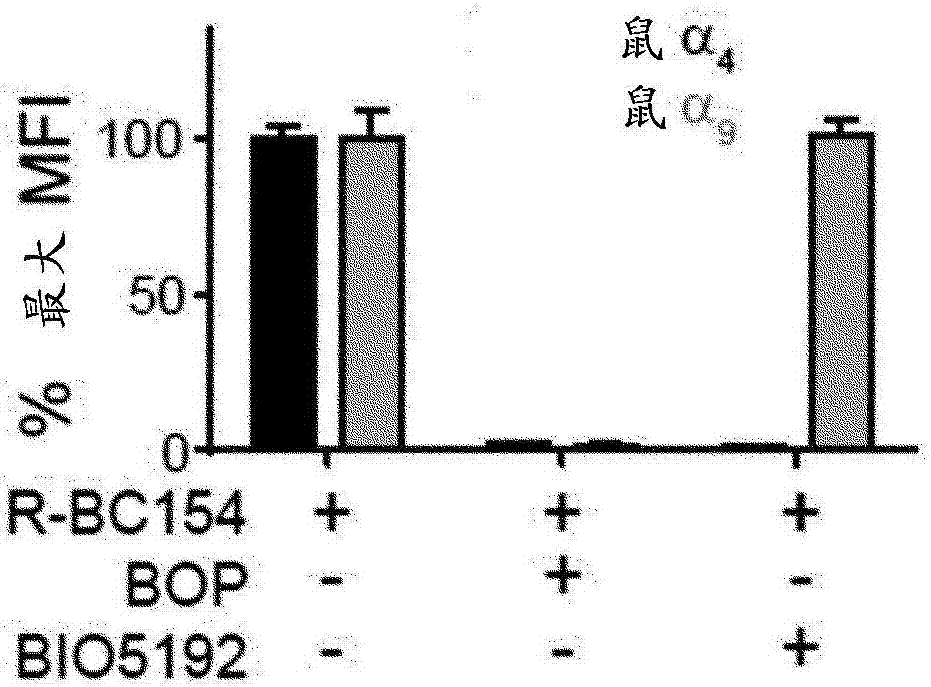
Dislodgement and release of HSC using alpha 9 integrin antagonist and CXCR4 antagonist
A technology of integrin and antagonist, applied in the direction of peptide/protein components, antineoplastic drugs, medical preparations containing active ingredients, etc., can solve problems such as toxic side effects and long treatment process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0484] Example 1: alpha 9 beta 1 Preparation of integrin antagonists
[0485] (a) Synthesis of Antagonist Compounds
[0486] The reagents of the various embodiments can be prepared using the reaction routes and synthetic schemes described below. The preparation of specific compounds of the embodiments is described in detail in the following examples, but the skilled artisan will recognize that the chemical reactions described can be readily adapted to prepare many other reagents of the various embodiments. For example, the synthesis of non-exemplary compounds can be successfully carried out by modifications apparent to those skilled in the art, for example, by appropriately protecting interfering groups, by changing to other suitable reagents known in the art, or by modifying the reaction conditions Make regular changes. A list of suitable protecting groups in organic synthesis can be found in T.W. Greene's Protective Groups in Organic Synthesis, 3rd edition, John Wiley & ...
Embodiment 1A
[0491] Example 1A: Preparation of N-(benzenesulfonyl)-L-prolyl-L-O-(1-pyrrolidinylcarbonyl)tyrosine (BOP)
[0492] The synthesis of BOP begins with dipeptide 26 as shown in Scheme 1 below:
[0493]
[0494] Process 1
[0495] Deprotection of the tert-butyl protecting group 26 using trifluoroacetic acid at 0°C afforded phenol 27, which was used in the next step without further purification after water workup. The reaction of phenol 27 with 1-pyrrolidinecarbonyl chloride proceeded smoothly in the presence of potassium carbonate, and the carbamate 28 was obtained in good yield (74%) in two steps. The hydrogenolysis of the Cbz protecting group was completed within 3 hours, The amine (85%) was obtained in excellent yield after flash chromatography. The amine 29 was then reacted with benzenesulfonyl chloride in the presence of base to give the sulfonamide 30 (96%) in excellent yield after flash chromatography. Finally, the methyl ester moiety 30 was saponified with sodium hydr...
Embodiment 1B
[0508] Example 1B: Preparation of R-BC154(IXb)
[0509] Compound IXb (R-BC154) lacking the PEG-spacer was also synthesized, as shown in Scheme 2 below:
[0510]
[0511] Process 2
[0512] Therefore, hydrolysis of formate 18 with NaOH gave the deprotected azide inhibitor 23, followed by 4 , sodium ascorbate and TBTA were reacted with N-propynylthiorhodamine B 24 to obtain a fluorescently labeled compound of formula IXb (R-BC154) in 43% yield after purification by HPLC.
[0513] As an example, the actual reaction conditions for the formation of the fluorescently labeled BOP derivative IXb starting from methyl ester 18 are provided herein.
[0514] Step 1: (S)-2-((2S,4R)-4-azido-1-(phenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-((pyrrolidine- 1-Carbonyl)oxy)phenyl)propionic acid (23)
[0515] Methyl ester 18 (420 mg, 0.737 mmol) in EtOH (10 mL) was treated with 0.2M NaOH (4.05 mL, 0.811 mmol) and stirred at room temperature for 1 hour. The mixture was concentrated under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com