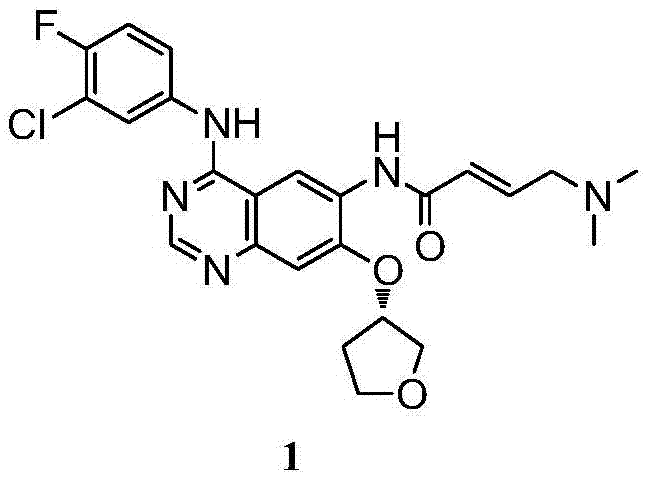
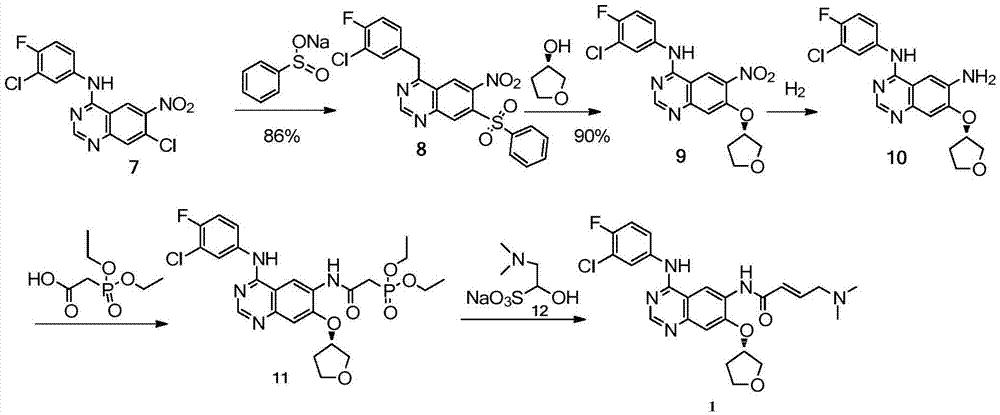
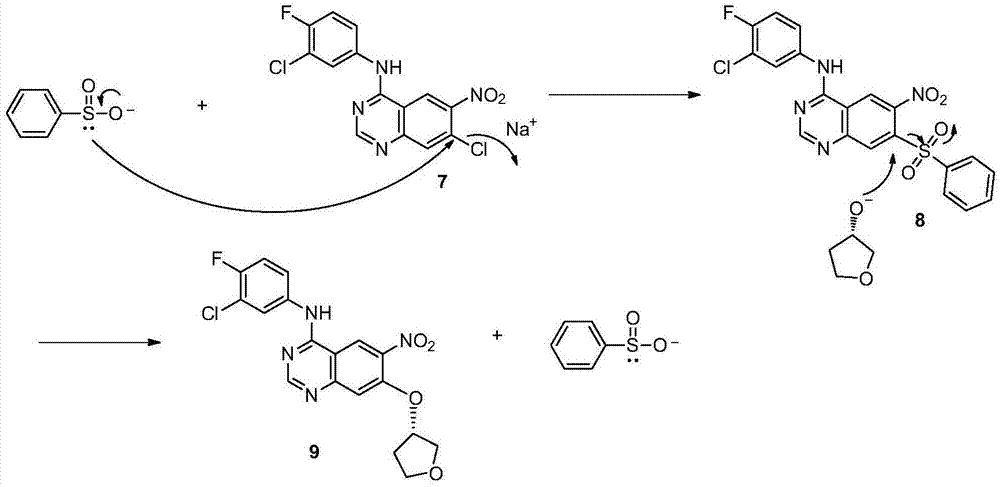
Method for preparing intermediate of Afatinib
A compound and molar ratio technology, applied in the field of preparation of afatinib intermediates, can solve the problems of unsuitable industrial production, complex operation, high cost, etc., and achieve the effect of less raw material consumption, simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 10 g of 6-nitro-4-[(3-chloro-4-fluoro-phenyl) amino]-7-chloroquinazoline and 0.57 g of sodium methylsulfinate into a three-necked flask equipped with a thermometer and a reflux condenser. g. (S)-3-hydroxytetrahydrofuran 5.2g, potassium carbonate 5.9g, DMF 50ml, react at 120°C for 3h. The reaction solution was added to 150 ml of water, filtered with suction, washed with water, and dried to obtain 7.0 g of black solid 9, with a yield of 61.2%. mp 243.1-249.9°C.
Embodiment 2
[0028] Add 20.0 g of 6-nitro-4-[(3-chloro-4-fluoro-phenyl)amino]-7-chloroquinazoline and sodium methylsulfinate into a three-necked flask equipped with a thermometer and a reflux condenser 1.27g, (S)-3-hydroxytetrahydrofuran 10.3g, potassium tert-butoxide 12.7g, DMF 100ml, react at 80°C for 3h. The reaction solution was added to 300ml of water, suction filtered, washed with water, and dried to obtain 21.3 g of yellow solid 9, with a yield of 93%. mp 245.2-247.9°C.
Embodiment 3
[0030] Add 20 g of 6-nitro-4-[(3-chloro-4-fluoro-phenyl)amino]-7-chloroquinazoline and 1.27 g of sodium methylsulfinate into a three-necked flask equipped with a thermometer and a reflux condenser. g. (S)-3-hydroxytetrahydrofuran 10.3g, potassium tert-butoxide 12.7g, DMF 100ml, react at 60°C for 12h. The reaction solution was added to 300ml of water, filtered with suction, washed with water, and dried to obtain 18.9 g of khaki solid 9, with a yield of 82.7%. mp 244.5-249.2°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com