Structural molecules that enhance integrin receptor affinity and target cell uptake and their applications
A technology of integrin receptors and affinity, applied in the field of biomedicine, can solve the problems of lack, incapacity of siRNA uptake and release ability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1 adopts the following steps:
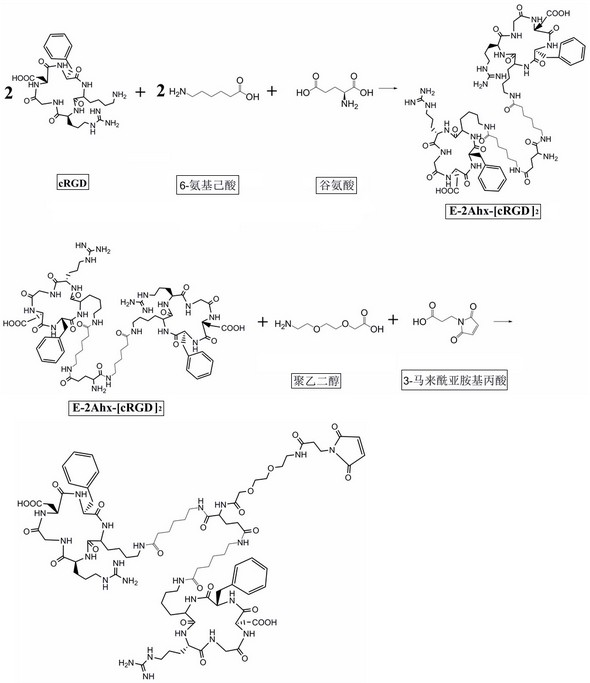
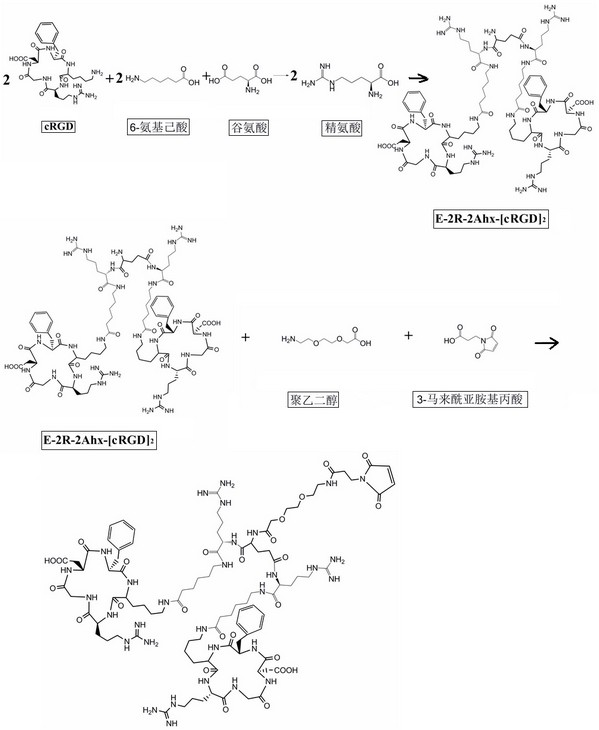
[0052] 1.1. Synthesize different molecular structures E-2Ahx-[cRGD]2-PEG-MPA (biRGD for short) and E-2R-2Ahx-[cRGD2-PEG-MPA (biRGD2 for short) that enhance the targeting affinity and uptake of tumor sites :
[0053] The synthesis steps of biRGD1 and biRGD2 are as follows: figure 1 and figure 2 As shown, the dehydration condensation of lysine in two monovalent cRGD motifs with 6-aminocaproic acid, glutamic acid and / or arginine, 8-amino-3,6-dioxahexanoic acid affords E-2Ahx -[cRGD]2-PEG or E-2R-2Ahx-[cRGD]2-PEG, then the amino group of PEG is dehydrated and condensed with the carboxyl group of 3-maleimidopropionic acid to obtain E-2Ahx-[cRGD]2 - PEG-MPA and E-2R-2Ahx-[cRGD]2-PEG-MPA.
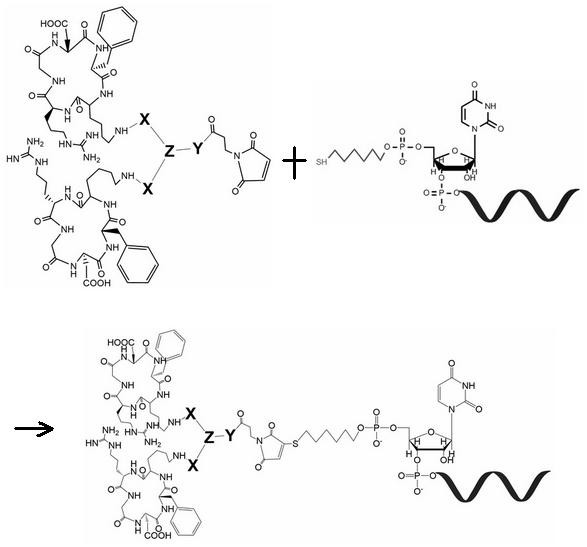
[0054] 1.2. Synthesis of positive-sense strand RNA with thiol (-SH) modification:
[0055] siACTB is a siRNA molecule that silences human β-actin mRNA and has undergone conventional stability modification; siNC is a siRNA molecule that has no...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com